FDA Awards HistoSonics Clearance of its First-of-a-Kind Edison® Histotripsy System
FDA Awards HistoSonics Clearance of its First-of-a-Kind Edison® Histotripsy System
Company Ramping Up Launch Plans of its “Breakthrough Device” Designated Platform
MINNEAPOLIS--(BUSINESS WIRE)--HistoSonics®, (www.histosonics.com), the manufacturer of the Edison® System and novel histotripsy therapy platforms, announced today the marketing authorization of its “Breakthrough” platform via the U.S. Food and Drug Administration's (FDA) De Novo Classification Request process, a rigorous pre-market review pathway for medical devices with no existing predicate. Marketing authorization makes Edison the first and only histotripsy platform available in the Unites States.
“This is HistoSonics’ most meaningful milestone to date and represents over two-decades of tireless efforts, from its inception in 2001, overcoming what was once thought to be impossible", commented Mike Blue, President, and CEO of HistoSonics.
Share
FDA authorization was based, in part, on data from the #HOPE4LIVER Trials in 13 trial sites across the US and Europe. Data pooled from both the US and European/UK trials were used to assess the clinical safety and efficacy of histotripsy in destroying targeted primary and secondary liver tumors. Histotripsy was noted to have achieved both primary safety and efficacy endpoints in the pooled data where 44 subjects were evaluated for safety and 44 tumors treated were evaluated for efficacy. Important to note was the heterogeneity of the treated subjects, 18 of which had hepatocellular carcinoma (HCC) tumors and 26 had metastatic tumors to the liver from the colon, rectum, breast, and other primary origins. As recently presented at the annual CIRSE Congress in Copenhagen, a technical success rate of 95.5% was achieved indicating that physicians can precisely target and destroy liver tissue and unresectable liver tumors. Also, only 3 procedure related CTCAE Grade 3 or higher adverse events through 30 days post-histotripsy were observed across all 44 subjects treated, representing a complication rate of 6.8% with each event being common to focal liver therapies and not specific to histotripsy.
“This is HistoSonics’ most meaningful milestone to date and represents over two-decades of tireless efforts, from its inception at the University of Michigan in 2001, overcoming what was once thought to be impossible - integrating the many complexities of histotripsy into a completely non-invasive clinical platform,” commented Mike Blue, President, and CEO of HistoSonics. The Company noted it has expanded its commercial and operational capacity over the previous year in preparation for commercial activities. Blue added, “We have been thoughtfully adding professionals with deep domain experience in operations, market development and education and are prepared to begin scheduling physician training immediately. This is a fantastic day for patients who will benefit from the novel advantages of histotripsy, and I commend the FDA for working so expeditiously with us throughout the review process.”
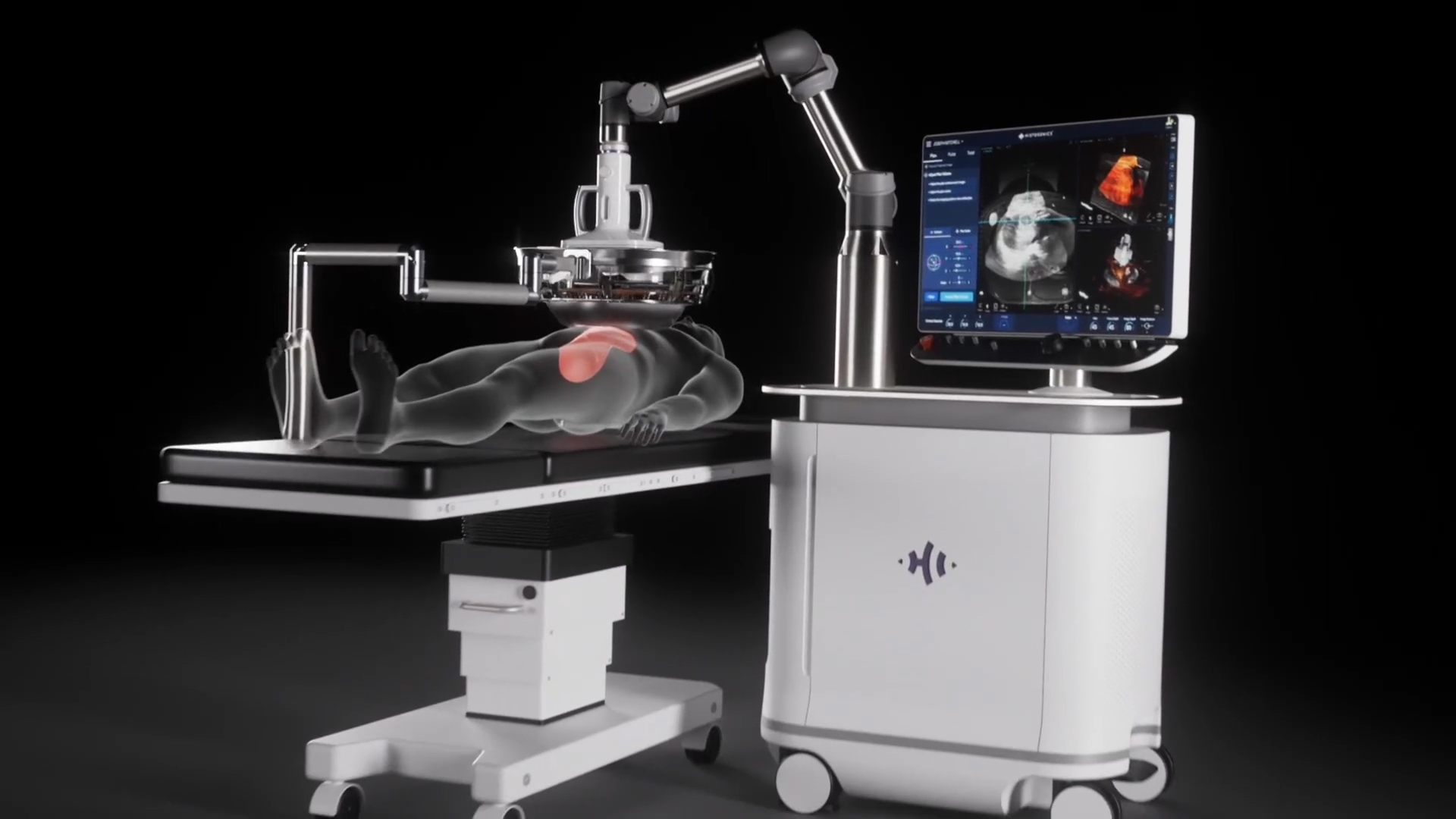
The science of histotripsy uses focused sound energy to produce controlled acoustic cavitation that mechanically destroys and liquifies targeted liver tissue, including tumors, at sub-cellular levels. HistoSonics' Edison System uses proprietary technology and advanced imaging to deliver personalized, non-invasive histotripsy treatments with precision and control. The company believes that the novel mechanism of action of their proprietary technology may provide significant advantages to patients, including the ability of the treatment site to recover and resorb quickly. Uniquely, the HistoSonics’ platform also provides physicians the ability to monitor the destruction of tissue under continuous real-time visualization and control, unlike any modality that exists today.
“As a surgeon, it’s rewarding to be able to offer a procedure where we can precisely destroy liver tumors without using a scalpel or needles, hopefully enabling the patient’s quick recovery while avoiding certain complications like surgical site infections or radiation illness common with other modalities,” commented Joe Amaral MD, VP Medical Affairs for HistoSonics. “Based on the data and patient experiences in our studies we are confident histotripsy will have a meaningful impact for patients suffering from unresectable liver disease, including liver tumors, and we look forward to the role histotripsy will play in treatment strategies going forward,” added Amaral.
The Edison System is indicated for the non-invasive destruction of liver tumors, including unresectable liver tumors, using a non-thermal, mechanical process of focused ultrasound.
About HistoSonics
HistoSonics is a privately held medical device company developing a non-invasive platform and proprietary sonic beam therapy utilizing the science of histotripsy, a novel mechanism of action that uses focused ultrasound to mechanically destroy and liquify unwanted tissue and tumors. The company is currently focused on commercializing their Edison System in the US and select global markets for liver treatment while expanding histotripsy applications into other organs like kidney, pancreas, and others. HistoSonics has offices in Ann Arbor, Michigan and Minneapolis, MN.
For more information please visit: www.histosonics.com/.
Contacts
Media contact:
Josh King
Vice President of Marketing
Email: Joshua.king@histosonics.com
Phone: 608.332.8124