Reflow Medical Receives CE Mark for Bare Temporary Spur Stent System for Treating de novo or Restenotic Below-the-Knee (BTK) Lesions
Reflow Medical Receives CE Mark for Bare Temporary Spur Stent System for Treating de novo or Restenotic Below-the-Knee (BTK) Lesions
SAN CLEMENTE, Calif.--(BUSINESS WIRE)--Reflow Medical, Inc., a developer of innovative medical devices focused on cardiovascular disease, announces it has received CE (Conformité Européenne) Mark certification in the European Union for the Bare Temporary Spur Stent System. The device is intended to treat de novo or restenotic lesions in the infrapopliteal arteries with a commercially available drug-coated balloon (DCB) to enhance drug absorption.
“The device performance and clinical study data for patients suffering from CLTI has been quite impressive,” said Professor Thomas Zeller, MD, who is Chief of the Department of Angiology at University Heart Center Freiburg in Bad Krozingen, Germany. CLTI, or chronic limb-threatening ischemia, increases the risk of mortality, amputation, and impaired quality of life. Prof. Zeller was a principal investigator in the DEEPER OUS clinical trial (NCT03807531).
The Bare Temporary Spur Stent System, followed by drug-coated balloon treatment, reduces clinically driven target lesion revascularization (CD-TLR), improves wound healing, reduces recoil, and improves vessel patency through one year, compared to historical treatment outcomes with plain balloon angioplasty or a DCB alone.
Marianne Brodmann, MD, is a Professor and Vascular Specialist with the Division of Angiology, Medical University Graz in Graz, Austria, and the Principal Investigator for the DEEPER LIMUS clinical trial (NCT04162418) to evaluate the Bare Temporary Spur Stent System. “We have found that the Spur allows us to treat patients with (BTK) disease using stent therapy, without the long-term risk of a stent implant,” she said.
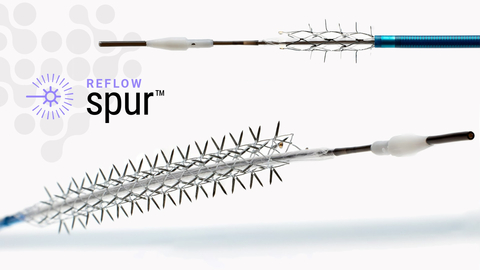
The Bare Temporary Spur Stent System is a unique clinical solution intended to provide stent-like results while leaving no metal behind. Known as Retrievable Stent Therapy, or RST, the self-expanding stent is designed with radial spikes for creating channels in the vessel wall to enhance drug absorption and reduce recoil. The stent is then recaptured, removed, and treated with a commercially available drug-coated balloon.
“Earning the CE Mark is a huge milestone for the company. It enables us to offer a clinically validated solution to an unmet need in a major disease area,” said Isa Rizk, Reflow Medical’s Co-Founder and CEO. “Our next goal is to expand our organization to commercialize this breakthrough technology and serve the needs of physicians and their patients in countries accepting this certification.”
The Reflow Medical Bare Temporary Spur Stent System was granted certification as a Class IIa medical device under the European Union Medical Device Regulation (2017/745). The EU MDR is a rigorous process that requires manufacturers to provide substantial evidence of their device’s performance and safety.
About Reflow Medical, Inc.
Reflow Medical, Inc., established in 2011, is a private company focused on empowering physicians through the design and development of innovative and effective technologies for treating cardiovascular disease.
Contacts
Jennifer Carlyle
jcarlyle@reflowmedical.com
949-481-0399