Maestro MEA Platform Powers First Stem Cell Cardiac Safety Assay in FDA ISTAND Program
Maestro MEA Platform Powers First Stem Cell Cardiac Safety Assay in FDA ISTAND Program
ATLANTA--(BUSINESS WIRE)--Axion BioSystems today announced that the FDA's Center for Drug Evaluation and Research (CDER) has accepted the company’s letter of intent (LOI) into the Innovative Science and Technology Approaches for New Drugs (ISTAND) Program for its Human iPSC-Cardiomyocyte MEA Assay for Prediction of Clinical Cardiovascular Repolarization Risk. The submission represents the first stem cell-derived, functional cardiac safety assay accepted into the ISTAND program.
“FDA acceptance marks an important milestone—not just for Axion BioSystems, but for the broader movement toward human-relevant cardiac safety assessment.”
Share
The ISTAND program is designed to advance promising, high-impact New Approach Methodologies (NAMs) through structured FDA engagement to inform regulatory decision-making. Acceptance of Axion’s LOI reflects the FDA’s interest in advancing mature, human-relevant, non-animal strategies that can strengthen early assessment of clinical cardiac risk. Cardiac safety remains a major challenge, contributing both to late-stage failures and, in some cases, the premature discontinuation of otherwise promising therapies.
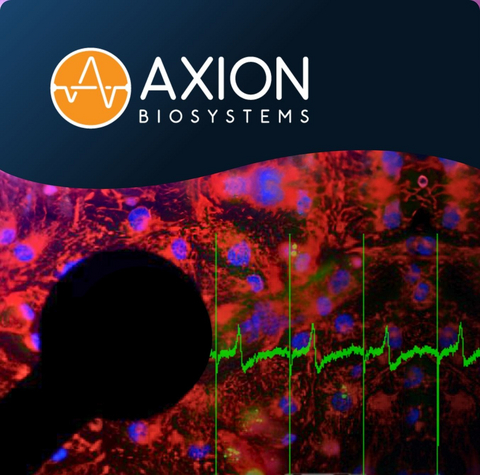
The proposed assay combines human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs) with Axion’s Maestro multielectrode array (MEA) technology to measure cardiac electrophysiology across a multicellular network, or syncytium. Using label-free, noninvasive recordings, the assay enables detection of delayed repolarization, beat-to-beat variability, and arrhythmia-like events associated with proarrhythmic risk—providing human-relevant functional insight beyond single-ion-channel or animal-based approaches.
Standardized implementation of the assay is enabled by Axion’s Maestro MEA platform, a widely adopted electrophysiology tool designed to support scalable, reproducible workflows in pharmaceutical and CRO environments. Maestro enables simultaneous recording across up to 96 wells with integrated environmental control, automation-ready operation, and multimodal capabilities. These features support consistent application of the hiPSC-CM MEA assay in regulatory-facing cardiac safety evaluations intended to inform decision-making.
“Acceptance into the FDA’s ISTAND Program is an important milestone, not just for Axion BioSystems, but for the broader movement toward human-relevant cardiac safety assessment,” said Julien Bradley, CEO of Axion BioSystems. “This assay reflects more than a decade of scientific validation, collaborative studies, and real-world use across industry and regulatory settings. We look forward to working with FDA to advance its qualification as a drug development tool that can increase confidence in early cardiac safety decisions.”
Axion has long played a key role in advancing functional cardiac safety assays, including participation in multicenter initiatives such as the Comprehensive in vitro Proarrhythmia Assay (CiPA). These efforts helped demonstrate that hiPSC-cardiomyocyte MEA assays can reliably identify delayed repolarization and arrhythmia-like events associated with torsades de pointes risk, contributing to their growing use in regulatory-facing drug development programs.
As part of the ISTAND program, Axion BioSystems will engage with FDA to develop a Qualification Plan defining the assay’s context of use, analytical validation strategy, and supporting evidence. Qualification of the assay as a DDT may enable broader regulatory acceptance of stem cell-derived functional cardiac safety data across drug development programs.
About Axion BioSystems
Axion BioSystems is a leading life sciences tools company focused on innovative live-cell assays used to study the function of cells in vitro for drug discovery and disease modeling. The team at Axion BioSystems is dedicated to continuing the advancement of new technologies that accelerate research and further the understanding of biological complexity outside of the body. Axion BioSystems is headquartered in Atlanta, Georgia, USA, and has offices in the Netherlands, the UK, and China.
Contacts
Mike Clements
SVP Scientific Partnerships & Strategy
+1-678-469-3439
mclements@axionbio.com