RevBio Receives a $2.4 Million NIH Grant to Conduct a First-in-Human Clinical Trial for its Regenerative Bone Adhesive for Cranial Flap Fixation and Enrolls First Five Patients
RevBio Receives a $2.4 Million NIH Grant to Conduct a First-in-Human Clinical Trial for its Regenerative Bone Adhesive for Cranial Flap Fixation and Enrolls First Five Patients
This Clinical Trial will Show Preliminary Safety and Probable Benefit of TETRANITE® for Replacing the Use of Metal Plates and Screws
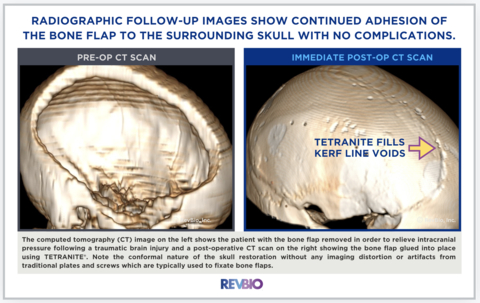
The computed tomography (CT) image on the left shows the patient with the bone flap removed to relieve intracranial pressure following a traumatic brain injury and a post-operative CT scan on the right showing the bone flap glued into place using TETRANITE. Note the conformal nature of the skull restoration without any imaging distortion or artifacts from traditional plates and screws which are typically used to fixate bone flaps. (Photo: Business Wire)
LOWELL, Mass.--(BUSINESS WIRE)--RevBio, Inc., announced that it has been awarded a $2.4 million Phase II Small Business Innovation Research (SBIR) grant from the National Institute of Neurological Disease and Stroke (NINDS), part of the National Institutes of Health (NIH). This two-year grant (1R44NS135736-01A1) will allow the company to complete a 20-patient pilot clinical trial to examine the safety and efficacy of the company’s bone adhesive biomaterial called TETRANITE® which will be used to immediately fixate cranial flaps and enable bone fusion following craniotomy procedures associated with brain surgery.
This clinical trial has already been approved by FDA for the stand-alone use of TETRANITE to replace metal plates and screws. The clinical trial is being conducted at the Semmes Murphey Clinic in Memphis, Tennessee, and Brigham and Women’s Hospital in Boston, Massachusetts. The first five patients have already been successfully enrolled, with the first patient having had more than 3-months of healing following the flap closure surgery involving TETRANITE. “Bone and soft tissue healing have progressed well, especially given the size of this bone flap,” said L. Madison Michael, MD, Co-Director of Cranial Base Surgery for the Methodist Brain and Spine Institute and practicing neurosurgeon at the Semmes Murphey Clinic in Memphis, Tennessee. “All radiographic follow-up images show continued adhesion of the bone flap to the surrounding skull with no complications.” Dr. Madison enrolled the first case in the study shown below.
“Advanced techniques have led to longer-term survival rates for patients who undergo brain surgery,” said Timothy R. Smith, MD, PhD, MPH, Director, Computational Neuroscience Outcomes Center and practicing neurosurgeon at Brigham and Women’s Hospital in Boston, Massachusetts. “Having just been through a very traumatic experience, improved bone healing through the revascularization of the flap and better cosmetic outcomes are important for patient self-esteem and for helping them move on with their lives”.
About RevBio, Inc.
RevBio, Inc., is a clinical stage medical device company engaged in the development and commercialization of a patented, synthetic, injectable, self-setting, and osteoconductive bone adhesive biomaterial called TETRANITE®. The company is initially developing this technology for use in the dental, cranial, and broader orthopaedic markets as well as applications in the animal health market. RevBio's TETRANITE technology is not yet approved for commercial use.
Contacts
Michael Tiedemann
mtiedemann@revbio.com
617-763-0923