FDA Greenlights RevBio’s Pivotal Clinical Trial for its Cranial Flap Bone Glue
FDA Greenlights RevBio’s Pivotal Clinical Trial for its Cranial Flap Bone Glue
This Important Milestone Represents the Initiation of the Final Development Phase Before Commercial Approval
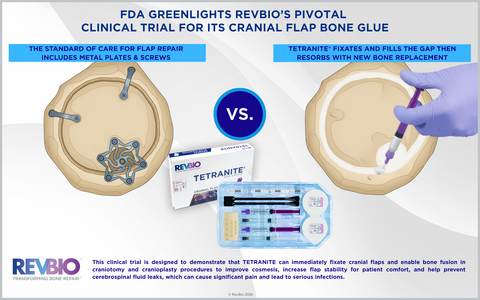
This clinical trial is designed to demonstrate that TETRANITE® can immediately fixate cranial flaps and enable bone fusion in craniotomy and cranioplasty procedures to improve cosmesis, increase flap stability for patient comfort, and help prevent cerebrospinal fluid leaks, which can cause significant pain and lead to serious infections.
LOWELL, Mass.--(BUSINESS WIRE)--RevBio, Inc., announced that it has received investigational device exemption (IDE) approval from the FDA to conduct a multi-center randomized controlled pivotal clinical trial to use TETRANITE® to reintegrate cranial bone flaps, which are portions of the skull that are temporarily removed by neurosurgeons to perform brain surgery.
“This novel regenerative adhesive has the potential to change how cranial restoration procedures are performed and significantly improve outcomes for patients,” said Timothy R. Smith, MD, PhD, MPH
Share
This clinical trial is designed to demonstrate that TETRANITE can immediately fixate cranial flaps and enable bone fusion in craniotomy and cranioplasty procedures to improve cosmesis, increase flap stability for patient comfort, and help prevent cerebrospinal fluid leaks, which can cause significant pain and lead to serious infections. All of these complications are known limitations of the current standard of care which consists of plate and screw fixation systems.
“This novel regenerative adhesive has the potential to change how cranial restoration procedures are performed and significantly improve outcomes for patients,” said Timothy R. Smith, MD, PhD, MPH, Director, Computational Neuroscience Outcomes Center and practicing neurosurgeon at Brigham and Women’s Hospital in Boston, Massachusetts.
This clinical trial, known as RevBio’s T-RESTORE II trial, will enroll up to 204 patients. Half of these patients will receive TETRANITE and the other half will receive standard cranial plate and screw systems to fixate their cranial bone flaps. Typically, craniotomy procedures are required to remove a brain tumor, treat an aneurysm, relieve intracranial pressure, or perform deep brain stimulation. Cutting into the skull leaves a kerf line, a continuous gap between the bone flap and surrounding skull. The flap is temporarily removed to conduct the brain surgery. In the surgical closure process, the cranial flap is secured back into place with titanium plates and screws. The kerf line, however, is typically not filled or sealed, which compromises the ability of the flap to integrate with the surrounding bone because the width of the kerf is too great a gap for bone cells to cross. As a regenerative bone adhesive placed into the kerf, TETRANITE immediately fixates the bone flap in its desired position, fills the gap, and provides a biological bridge for bone cells to travel from the surrounding skull. This allows bone fusion to occur and the bone flap to solidly heal.
The FDA approved up to 15 clinical sites to participate in this study. Currently, RevBio is actively recruiting sites based on feedback from its neurosurgical advisory board members. This approval was largely predicated on the successful pivotal preclinical study, surgeon handling testing, and successful pilot clinical trial (T-RESTORE I) conducted by the company.
“This pivotal IDE trial allows us to conduct the final testing required for the commercial approval of our lead indication in the $10 billion market of targeted bone glue applications,” said Brian Hess, CEO and founder of RevBio, Inc. “The fact that RevBio now has two active pivotal stage programs highlights our ability to scale this technology as a platform.”
About RevBio, Inc.
RevBio, Inc., is a clinical stage medical device company engaged in the development and commercialization of TETRANITE®, a patented, synthetic, injectable, self-setting, and osteoconductive bone adhesive. The company is initially developing this technology for use in the dental, cranial, and broader orthopaedic markets as well as applications in the animal health market. RevBio's TETRANITE technology is not yet approved for commercial use.
Contacts
Michael Tiedemann
mtiedemann@revbio.com