Realeve Unveils Breakthrough Solution to Treat Central Nervous System Disorders Including Stroke and Cluster Headache; Bypasses Brain’s Natural Barrier to Deliver Therapeutics
Realeve Unveils Breakthrough Solution to Treat Central Nervous System Disorders Including Stroke and Cluster Headache; Bypasses Brain’s Natural Barrier to Deliver Therapeutics
Company receives multimillion dollar grant to focus on post-stroke recovery; Proven technology in 700 patients granted Breakthrough Device designation from the FDA
EFFINGHAM, Ill.--(BUSINESS WIRE)--Realeve today announced it has received a multimillion dollar grant to support a study at Cleveland Clinic aimed at revolutionizing post-stroke recovery. The planned study will use Realeve’s Pulsante® micro-neuromodulation system, the first externally powered implantable neuromodulation device which stimulates the sphenopalatine ganglion (SPG), in conjunction with hyperbaric oxygen to potentially enhance blood circulation in stroke patients, minimizing brain damage and optimizing neurological recovery.
Realeve’s technology has already been clinically proven in more than 700 patients for the treatment of a brain related disorder. Realeve has also been granted Breakthrough Device designation from the FDA with a goal of obtaining FDA approval and CE Mark to treat cluster headaches in 2024. Breakthrough Device designations are given by the U.S. FDA to expedite the review of technologies that can improve the lives of people with life-threatening or debilitating conditions.
“We are excited to leverage the power of this external implant that has been clinically proven with 700+ patients in enhancing post-stroke and cluster headache recovery. We are also exploring its applications in other central nervous system disease conditions and excited about the potential,” states Dr. Peter Bonutti, founder and president, Realeve.
Realeve’s ultimate goal is to solve one of the critical remaining barriers in brain health which is the ability to bypass the brain's natural barrier preventing the delivery of effective drugs for stroke, cancer treatment, and other degenerative orders. Realeve is evaluating the potential effects of its patented SPG therapy on the Blood Brain Barrier (BBB) using its clinically proven Pulsante System. The BBB functions as a vascular gatekeeper to the central nervous system (CNS), governing the passage of medications and therapeutic interventions into the brain. This technology holds promise for addressing conditions like Alzheimer's, tumors, and other central nervous system disorders. The convergence of these therapies is a potentially significant advancement in treating neurological diseases of the brain, and a compelling opportunity for investors in the biotech sector.
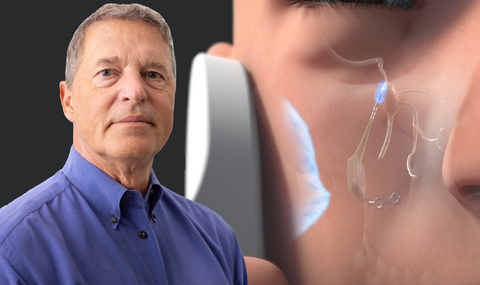
How Pulsante Works:
Pulsante consists of a miniature bioactive implant inserted orally using a minimally invasive technique that leaves no visible scars. It pairs with an external, hand-held controller that stimulates the SPG, which controls the autonomic nervous system, which can impact multiple neurological conditions. The targeted therapy provides both pain relief, and can increase blood flow and medication delivery to the central nervous system.
Additionally, Realeve has solved a key challenge with surgical implants: powering them. Most implants such as pacemakers, cardiac and insulin pumps, etc. require batteries. These batteries have many challenges, the biggest is that batteries wear out which requires an additional surgery. The Pulsante handheld remote control wirelessly powers the implant and allows for two-way communication and wireless updates to the system.
The Realeve medical team is led by Dr. Peter Bonutti, a renowned orthopedic surgeon, inventor, author, and professor with more than 500 patents, and Dr. Frank A. Papay, M.D., Chairman, Dermatology and Plastic Surgery Institute, Cleveland Clinic Professor, Lerner College of Medicine at Case Western Reserve University.
“Realeve is focused on optimizing therapies to the central nervous system. With a commitment to innovation and a patient-centric approach, the company aims to improve the lives of individuals with neurological conditions through stimulating extra cranial nerves. By developing novel technology, Realeve strives to provide effective, targeted relief that can make a lasting impact on patient well-being,” said Dr. Frank A. Papay, Realeve Advisory Board.
Dr. Papay is an inventor of technology that Cleveland Clinic licensed to Unity, HA, the parent company of Realeve. He and Cleveland Clinic are entitled to royalties and other commercialization revenues related to the technology. In addition, Dr. Papay serves as a consultant to the company, for which he received equity.
About Realeve, LLC:
Realeve is a medical device company focused on developing novel treatments for diseases and conditions of the CNS by controlling the SPG via the Pulsante micro-neurostimulator system. Founded in 2019, Realeve builds on over a decade of safe and proven efficacy of the Pulsante system. For more information, visit realeve.net.
Investigational device. Limited by Federal (or United States) law to investigational use.
Contacts
Donna Loughlin Michaels for Realeve
LMGPR
donna@lmgpr.com
(408) 393-5575