Attune Medical’s ensoETM™ Granted FDA De Novo Marketing Authorization to Reduce the Likelihood of Ablation-related Esophageal Injury Resulting from Radiofrequency Cardiac Ablation Procedures
Attune Medical’s ensoETM™ Granted FDA De Novo Marketing Authorization to Reduce the Likelihood of Ablation-related Esophageal Injury Resulting from Radiofrequency Cardiac Ablation Procedures
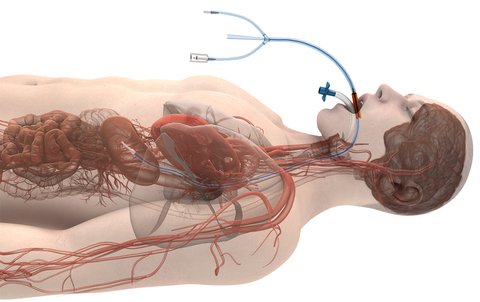
Attune Medical has been granted De Novo marketing authorization from the US Food and Drug Administration (FDA) for its ensoETM device to reduce the likelihood of ablation-related esophageal injury resulting from radiofrequency cardiac ablation procedures. The ensoETM is the pioneer of using the esophageal space to manage temperature and has been cleared for use for the control of patient temperature since 2015. Over 50,000 patients have been treated with the ensoETM to date in critical care units, emergency rooms, operating rooms, and electrophysiology labs. (Graphic: Business Wire)
CHICAGO--(BUSINESS WIRE)--Attune Medical has been granted De Novo marketing authorization from the US Food and Drug Administration (FDA) for its ensoETM device to reduce the likelihood of ablation-related esophageal injury resulting from radiofrequency cardiac ablation procedures.
The FDA based its decision on pre-clinical studies, computer models, three randomized controlled studies, and data on tens of thousands of patients treated in real-world uses. Published studies that have examined the use of ensoETM for this purpose include the pilot eCOOL-AF study, the IMPACT study and a large multi-center analysis of over 25,000 patients (in press at the Journal of the American College of Cardiology: Clinical Electrophysiology). These studies have found up to an 83 percent reduction in esophageal injuries, and a significant reduction in risk of atrioesophageal fistula (AEF), one of the most dreaded complications of ablation procedures.
Cardiac ablation utilizing radiofrequency energy is used to restore normal heart rhythm in patients with atrial fibrillation by disrupting faulty electrical pathways that interfere with normal rhythm. It is the most common type of cardiac ablation procedure, with over 300,000 per year performed in the US alone.
Commonly available technologies to mitigate serious complications have not been successful in reducing the risk of catastrophic esophageal injuries in over 20 years of use. This De Novo marketing authorization expands the ensoETM’s indications for use to reduce the likelihood of esophageal injury resulting from radiofrequency cardiac ablation procedures. The ensoETM is the pioneer of using the esophageal space to manage temperature and has been cleared for use for the control of patient temperature since 2015. Over 50,000 patients have been treated with the ensoETM to date in critical care units, emergency rooms, operating rooms, and electrophysiology labs.
“Historically, there have been no proven strategies to prevent esophageal injury during ablation procedures, and injury rates have not declined despite the use of temperature probes,” commented Jason Zagrodzky, MD, FHRS, Electrophysiologist at Texas Cardiac Arrhythmia in Austin, Texas. “This De Novo authorization gives electrophysiologists a solution to proactively cool the esophageal wall during ablation procedures and is a great leap forward in best practice standards and patient care. We have found a 35 percent reduction in fluoroscopy requirements with cooling, as well as a reduced staff workload which results in significant cost savings to hospital systems.”
“Our lab has published studies showing a 30 percent reduction in procedure time and a 14 percent improvement in long-term efficacy when using ensoETM,” noted Mark Metzl, MD, FHRS, Section Chief, Cardiac Electrophysiology at NorthShore University Health System. “These findings have in turn been shown to result in a quicker return home for patients and a cost savings of up to $2,135 per procedure.”
“Over the last 20 years, significant resources have been committed to mitigating serious esophageal complications, with no meaningful results. This De Novo marketing authorization opens the door to a new standard. Studies have shown improved safety and efficacy for patients while allowing improved efficiency for physicians and greater cost savings for the hospitals in which they operate,” commented Jay Istvan, Attune Medical’s CEO.
Attune Medical’s ensoETM is a single use thermal regulating device that is placed in the esophagus (similar to a standard orogastric tube) and connected to an external heat exchange unit, creating a closed-loop system for proactive controlled temperature management.
ABOUT ATTUNE MEDICAL
Attune Medical’s novel medical device technology pioneered the practice of using the esophageal space to proactively manage patient temperature and to reduce the likelihood of esophageal injury resulting from radiofrequency cardiac ablation procedures.
Contacts
Lisa Owens, lisammowens@gmail.com, 210.601.6647