Paige Receives First Ever FDA Approval for AI Product in Digital Pathology
Paige Receives First Ever FDA Approval for AI Product in Digital Pathology
Paige Prostate was granted de novo marketing authorization from the FDA to aid in the primary diagnosis of prostate cancer
NEW YORK--(BUSINESS WIRE)--Paige, the global leader in AI-based diagnostic software in pathology, today announced that the U.S. Food and Drug Administration (FDA) has granted de novo marketing authorization for Paige Prostate, a clinical-grade AI solution for prostate cancer detection. As a novel technology, Paige Prostate is the first AI-based pathology product to receive de novo approval from the FDA, allowing in vitro diagnostic (IVD) use via Paige’s FDA-cleared FullFocus™ digital pathology viewer.
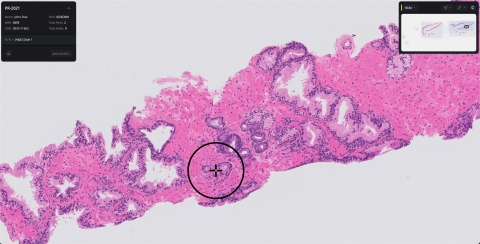
With a projected 60 percent increase in the number of cancer cases globally in the next two decades and a decrease in the number of pathologists relative to this diagnostic demand, there is a significant need to provide new technologies for the practice of pathology. Paige Prostate is a cancer detection solution that identifies foci suspicious for cancer and provides this information to the pathologist. Paige Prostate is designed to assist pathologists in finding small foci of cancer and enable pathologists to work efficiently and confidently in their diagnostic process.
“This landmark approval of Paige Prostate by the FDA marks the beginning of a new era in the use of computer-assisted diagnostics for pathology. The approval reflects the rigor with which Paige Prostate has been validated, as the first clinical-grade AI technology to assist pathologists in the interpretation of routinely stained slides. This innovation paves the way for the introduction of numerous future tools to help standardize pathology diagnosis, expedite the diagnostic process, and provide pathologists and patients greater comfort from the added scrutiny of their pathology slides,” said David Klimstra, M.D., Co-Founder and Chief Medical Officer at Paige. “FDA approval allows pathology laboratories to introduce this diagnostic tool into their clinical workflow to help make pathologists more accurate, more reproducible, and more efficient, which will allow them to focus their attention on the most critical aspects of establishing the diagnosis.”
“This achievement is the culmination of more than a decade of work and a testament to the dedication of Paige and our collaborators in developing clinical-grade AI that will transform the practice of pathology,” said Thomas J. Fuchs, Dr.Sc., Co-Founder and Chief Scientist at Paige, and Dean of Artificial Intelligence and Human Health at Mount Sinai.
In the clinical study submitted to the FDA, pathologists using Paige Prostate were shown to increase over 7 percentage points in sensitivity in correctly diagnosing cancer (from 89.5% to 96.8%). Pathologists using Paige Prostate had a 70% reduction in false negative diagnoses and a 24% reduction in false positive diagnoses. This improvement was independent of diagnostic sub-specialization or years of experience of the pathologists and whether the analysis was done remotely or on-site. Furthermore, the study showed that non-specialist pathologists using Paige Prostate were as accurate as prostate specialists who were not using the software. The dataset included slides from over 150 institutions to ensure the system generalized to cases from different hospitals and different geographies. This dataset represented a broad range of natural variations encountered in day-to-day clinical practice and Paige Prostate was used across this entire dataset without modification or the need for calibration. The company plans to submit the full data for publication in a peer-reviewed journal.
“The approval is a landmark achievement in the field of digital pathology and demonstrates how robust our technology is when faced with the broad range of natural variations in tissue slides encountered in day-to-day clinical practice,” said Leo Grady, Ph.D., Chief Executive Officer of Paige. “We are grateful for everyone at Paige and our clinical partners who have brought this new generation of computational pathology products to reality.”
Paige Prostate is now available for diagnostic use in the U.S. For more information about Paige Prostate, visit https://info.paige.ai/prostate or contact info@paige.ai.
Outside of the U.S., Paige Prostate is CE-marked for use in laboratories and hospitals in the European Economic Area, Switzerland and the UK. FullFocus is FDA cleared and CE-marked. The products are otherwise available for research use only in other territories.
About Paige
Paige was founded in 2017 by Thomas Fuchs, Dr.Sc., David Klimstra, M.D. and colleagues from Memorial Sloan Kettering Cancer Center (MSK). The company builds computational pathology products designed so patients and their care teams can make effective, more informed treatment decisions. With this new class of AI-based technologies positioned to drive the future of diagnostics, Paige created a platform to deliver this novel technology to pathologists to transform their workflow and increase diagnostic confidence and productivity. Paige’s products deliver insights to pathologists and oncologists so they can arrive efficiently at more precise diagnoses for patients. Paige is the first company to receive FDA approval for an AI-based digital pathology product.
For additional information, please visit: https://www.paige.ai, Twitter and LinkedIn.
Contacts
For Media:
Jon Yu
Westwicke/ICR Healthcare PR
Tel: 475.395.5375
Jon.Yu@westwicke.com