Novotech Analysis Highlights Rising Clinical Development Efforts for Obesity Treatments
Novotech Analysis Highlights Rising Clinical Development Efforts for Obesity Treatments
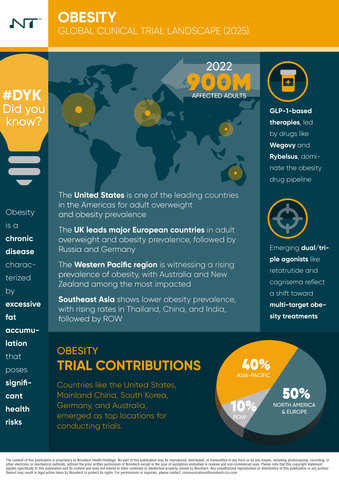
SYDNEY--(BUSINESS WIRE)--Novotech, a leading global clinical research organization (CRO) and scientific advisory partner, has published a report analyzing recent trends in obesity-related clinical trials. The report shows that the number of these trials tripled between 2019 and 2024, reflecting increased research activity and broader range of potential therapeutic options for obesity treatments.
Novotech, a leading global clinical research organization published a report analyzing recent trends in obesity-related clinical trials. The report shows that the number of these trials tripled between 2019 and 2024.
Share
Obesity continues to represent a major global health challenge, affecting nearly 900 million adults worldwide and placing a substantial burden on healthcare systems and quality of life. Strongly linked to conditions such as type 2 diabetes, cardiovascular disease, musculoskeletal disorders, and certain cancers, obesity demands targeted and innovative therapeutic strategies.
The report provides biotech and pharmaceutical companies that engage in obesity-related therapies a comprehensive analysis of emerging therapeutic strategies, biomarker research developments, and global funding trends. As obesity rates continue to rise across all regions, the report provides actionable market insights and strategic considerations to support companies in advancing their obesity treatment pipeline and shaping informed pathways towards successful product development and commercialization.
With deep therapeutic expertise, extensive regulatory knowledge, and access to over 5,000 clinical trial sites globally, Novotech remains committed to accelerating the clinical development process. Leveraging comprehensive local market insights and advanced analytical capabilities, Novotech supports biotech and pharmaceutical innovators in efficiently navigating complex obesity trials.
Download the full Global Clinical Trial Landscape report on obesity here.
For further information, please visit novotech-cro.com
About Novotech Novotech-CRO.com
Novotech is a globally recognized full-service clinical research organization (CRO) and scientific advisory company trusted by biotech and small- to mid-sized pharmaceutical companies to guide drug development at every phase.
With a global footprint that includes 30+ offices across the Asia-Pacific region, North America, and Europe and partnerships with 5,000+ trial sites, Novotech provides clients an accelerated path to bring life-changing therapies to market by providing access to key clinical trial destinations and diverse patient populations.
Through its client-centric service model, Novotech seamlessly integrates people, processes, and technologies to deliver customized solutions that accelerate the path to market for life-changing therapies. By adopting a true partnership approach, Novotech shares a steadfast commitment to client success, empowering innovation, and advancing healthcare worldwide.
Recipient of numerous industry accolades, including the Frost & Sullivan CRO Company of the Year award for 19 consecutive years, Novotech is recognized for its excellence in clinical trial execution and innovation. Its deep therapeutic and regulatory expertise, combined with local market insights, ensures streamlined clinical trials, optimized data analytics, and accelerated patient recruitment strategies.
Together with clients, Novotech transforms scientific advancements into therapies that improve global health outcomes, embodying a mission of driving innovation and delivering impactful results.
For more information or to speak to an expert team member visit www.Novotech-CRO.com
Contacts
Media Contact
Toyna Chin
mediacontact@novotech-cro.com
USA: +1 415 364 8135