Novotech Issues White Paper to Help Sponsors Strengthen Early-Phase Oncology Strategy and Execution
Novotech Issues White Paper to Help Sponsors Strengthen Early-Phase Oncology Strategy and Execution
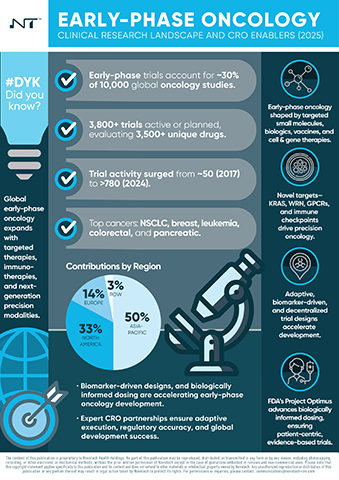
SYDNEY--(BUSINESS WIRE)--Novotech, a leading global full-service clinical research organization (CRO) and scientific advisory company, has released a new white paper, Early-Phase Oncology – Clinical Research Landscape and CRO Enablers (2025), offering in-depth analysis of the key drivers shaping early-phase oncology development.
With approximately 60% of oncology agents progressing from Phase I to Phase II, but only 3–6% reaching regulatory approval, the paper outlines the factors influencing early-stage success, including trial design, patient selection, and regional development pathways.
The paper highlights Australia’s continued position as a global first-in-human (FIH) and early-phase research hub. Its ethics-led review frameworks, which can support trial initiation within roughly 4–8 weeks, combined with established FIH centers, oncology networks, and cost efficiencies, position Australia as a practical starting point for programs that later expand into the US, Europe, and broader Asia markets.
The analysis provides biotech and pharmaceutical sponsors with analysis of how modern trial methodologies, precision-based approaches, and coordinated global execution are shaping early-phase oncology. It also highlights Novotech’s role in supporting these programs through scientific expertise, regulatory alignment, and access to more than 5,500+ trial sites worldwide.
Key areas covered:
- Global Early-Phase Oncology Landscape: Geographic trends, therapeutic innovation, and investment momentum driving new oncology pipelines.
- Emerging Trial Designs: Adaptive, biomarker-driven, and decentralized models improving speed, inclusivity, and data quality.
- Regulatory Shifts: The impact of initiatives such as the U.S. FDA’s Project Optimus in advancing evidence-based, patient-centric dosing strategies.
- Novotech’s Role as a CRO Partner of Choice: The organization’s early-phase oncology expertise and technology-driven model help sponsors manage risk, shorten timelines, and strengthen data quality.
Download the white paper: Early-Phase Oncology – Clinical Research Landscape and CRO Enablers (2025)
About Novotech Novotech-CRO.com
Novotech is a globally recognized full-service clinical research organization (CRO) and scientific advisory company trusted by biotech and small- to mid-sized pharmaceutical companies to guide drug development at every phase.
With a global footprint that includes 30+ offices across the Asia-Pacific region, North America, and Europe and partnerships with 5,000+ trial sites, Novotech provides clients an accelerated path to bring life-changing therapies to market by providing access to key clinical trial destinations and diverse patient populations.
Through its client-centric service model, Novotech seamlessly integrates people, processes, and technologies to deliver customized solutions that accelerate the path to market for life-changing therapies. By adopting a true partnership approach, Novotech shares a steadfast commitment to client success, empowering innovation, and advancing healthcare worldwide.
Recipient of numerous industry accolades, including the Frost & Sullivan CRO Company of the Year award for 19 consecutive years, Novotech is recognized for its excellence in clinical trial execution and innovation. Its deep therapeutic and regulatory expertise, combined with local market insights, ensures streamlined clinical trials, optimized data analytics, and accelerated patient recruitment strategies.
Together with clients, Novotech transforms scientific advancements into therapies that improve global health outcomes, embodying a mission of driving innovation and delivering impactful results
For more information or to speak to an expert team member visit www.Novotech-CRO.com
Contacts
Media Contact
Toyna Chin
mediacontact@novotech-cro.com
USA: +1 415 364 8135