FDA Grants Breakthrough Device Status to Toku’s Patented Cardiovascular Risk AI (CLAiR) Platform
FDA Grants Breakthrough Device Status to Toku’s Patented Cardiovascular Risk AI (CLAiR) Platform
Toku’s AI (CLAiR) technology platform has received FDA Breakthrough Device status, and if cleared by the FDA will deliver real-time cardiovascular disease (CVD) risk assessments through routine eye exams. FDA's Breakthrough Device designation expedites the development and review process, shortening the time until Toku's AI technology is able to reach the market. Consequently, patients, especially those in lower socioeconomic groups who may lack access to robust healthcare, will benefit from earlier access to non-invasive and point-of-care CVD risk assessments, which in turn can lead to early prevention and improved outcomes. The patented CLAiR AI technology platform, if cleared for marketing, will integrate seamlessly into existing retinal cameras to allow widespread access to CVD assessments through eye care clinics, primary care, and pharmacies. Toku recently joined the Innovator’s Network of the American Heart Association’s Center for Health Technology & Innovation, a group dedicated to fostering development of innovative and scalable healthcare solutions. Toku plans to reach the US market by mid-2025.
AUCKLAND, New Zealand--(BUSINESS WIRE)--Toku, Inc., a commercial medical device company specializing in imaging technology and AI, announced that the U.S. Food and Drug Administration (FDA) has granted Breakthrough Device designation to its patented CLAiR technology. The CLAiR platform, if cleared by the FDA, will be the first medical device in the US market that can provide affordable, point-of-care and non-invasive evaluation for risk of cardiovascular disease (CVD) using fundus retinal images through a routine eye exam. Working with its partners, Toku is aiming to establish the largest network for CVD risk assessment across the US and then globally. Toku recently joined the Innovator’s Network, part of the American Heart Association’s Center for Health and Technology & Innovation. Inclusion in this group will enable Toku to collaborate easily with other cutting-edge companies creating the next generation of high-tech healthcare solutions as the Company works to bring the CLAiR platform to scale commercially, pending clearance from the FDA.
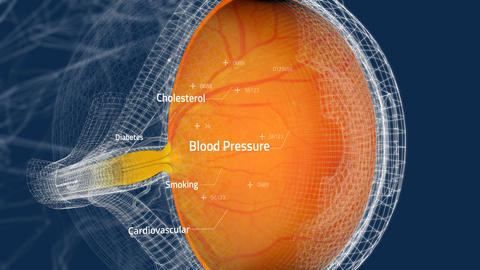
The retina, located in the back of the eye, is the only transparent part of the vascular system and can be photographed easily and non-invasively. The CLAiR technology is designed to integrate readily with existing retinal imaging cameras, to provide real-time CVD risk assessments with accuracy comparable to traditional cardiovascular risk assessment tools (which typically include multiple measurements and blood tests and can take weeks). The AI-powered CLAiR technology can interpret the many tiny signals conveyed through retinal images of blood vessels to identify elevated cardiovascular risk that may be caused by genetics or risk factors such as hypertension or high cholesterol. These results can then be shared with the patient’s primary care physician, who can initiate a comprehensive cardiovascular evaluation. Retinal imaging is routinely performed in a variety of eyecare settings and is increasingly being implemented in primary care clinics and pharmacies across the US. Once cleared by the FDA, the CLAiR technology will provide healthcare professionals across multiple settings with the ability to check for elevated cardiovascular risk before the onset of clinical disease.
“Toku’s mission is to make identifying disease accessible for everyone, everywhere, all the time. The Breakthrough Device designation that the FDA has granted to our CLAiR technology platform is a validation of the tremendous potential our CLAiR AI technology can provide to the tens of millions of patients who may unknowingly be at risk of a devastating cardiovascular condition,” said Associate Professor Ehsan Vaghefi, CEO and Co-Founder of Toku. “This designation greatly de-risks our clinical development and regulatory pathway for the technology, as the FDA’s Breakthrough Devices program offers medical device companies accelerated review processes, enhanced guidance, and prioritized evaluation, facilitating quicker market access for innovative technologies and encouraging the development of devices that significantly improve patient care.”
“I am excited by the potential of Toku’s CLAiR technology as it can help improve health equity both in the United States and elsewhere in the world,” said Michael V. McConnell, MD, MSEE, Clinical Professor of Preventive Cardiology, Stanford University and author of Fight Heart Disease Like Cancer. “As a clinician, I see broadening access to quality care and prevention as a critical issue, and the CLAiR retina scan technology may help improve the cardiovascular health of more people worldwide.”
“Predictive analytics technology, provided it has been proven to be accurate and applicable to the population using it, has tremendous potential to benefit patients by identifying those at highest risk so treatments and preventive measures can be initiated quickly. The medical literature has published many examples using different types of eye imaging to predict risk of other systemic conditions beyond cardiovascular disease, such as neurologic or kidney disease. Therefore, further developing this type of technology is very exciting as cardiovascular disease remains a significant cause of morbidity and mortality in the whole country,” said April Maa, MD, Professor of Ophthalmology, Emory University School of Medicine.
About Toku, Inc.
Toku, Inc. is a cutting-edge technology company that specializes in developing non-invasive, AI-powered diagnostic and screening tools using retinal imaging to measure cardiovascular and other health risk factors. The company’s first commercialized product, the BioAge Test, uses advanced imaging techniques to analyze various biometric markers in the body to accurately measure an individual’s biological age. The test can provide a detailed report on an individual’s overall health and potential health risks. The Company is also developing the CLAiR platform, an investigational, AI-powered technology to assess cardiovascular risk by means of fundus retina scans of the blood vessels in the back of the eye. The CLAiR platform has received Breakthrough Device designation from the FDA and Toku is committed to making its technology widely accessible through its major partnerships across the world. The company’s technology is built on the latest research in artificial intelligence, and its team of experts includes leading scientists and medical professionals.
Contacts
Media Inquiries:
Harriet Ullman
Vice President
LaVoieHealthScience
T: +1 617.429.5475
hullman@lavoiehealthscience.com