CMS Grants HCPCS Code and NTAP Classification for Pleural Dynamics’ Novel ACES Automatic Continuous Effusion Shunt
CMS Grants HCPCS Code and NTAP Classification for Pleural Dynamics’ Novel ACES Automatic Continuous Effusion Shunt
MINNEAPOLIS--(BUSINESS WIRE)--The Centers for Medicare and Medicaid Services (CMS) has issued a new Healthcare Common Procedure Coding System (HCPCS) code and New Technology Ambulatory Payment Classification (New Technology APC) assignment for the ACES (Automatic Continuous Effusion Shunt) device to treat chronic pleural effusions. The new code issued is C8006: Insertion of pleural-peritoneal shunt with intercostal pump chamber, including imaging, injection(s) of contrast with radiological supervision and interpretation, when performed and will be paid at a commensurate reimbursement level to support usage of the ACES device.
“With this reimbursement pathway, hospitals will now be better positioned to offer ACES, ensuring that more patients have access to a technology designed to improve outcomes, reduce repeat interventions, and enhance quality of life.” - Martin Mayse, M.D.
Share
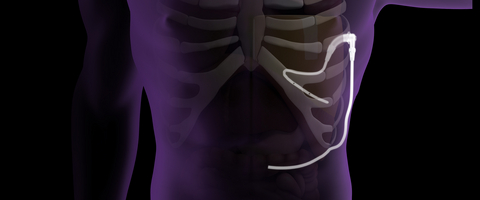
The ACES Automatic Continuous Effusion Shunt System is the first and only fully implantable automatic effusion shunt that uses the motion of normal breathing to remove fluid from the chest. It is designed for continuous symptom relief and does not require an extended hospital stay, a catheter external to the chest, or expensive drainage canisters. The ACES System received FDA 510(k) clearance in August 2023 and is in early market release in the United States.
“We commend CMS for creating an HCPCS code and granting a New Technology APC assignment for the ACES™ implant, an important step that validates both the clinical and resource needs associated with this novel procedure,” said Martin Mayse, M.D., CEO and Co-Founder of Pleural Dynamics. “With this reimbursement pathway, hospitals will now be better positioned to offer ACES, ensuring that more patients have access to a technology designed to improve outcomes, reduce repeat interventions, and enhance quality of life.”
The current standards of care for recurrent pleural effusion (PE) have several significant shortcomings for patients. One standard approach for treating recurrent PE’s is pleurodesis, which is painful, requires an extended hospital stay, and is often unsuccessful, requiring additional procedures.1 A common alternate approach—indwelling pleural catheters—requires a portion of the catheter to be external to the chest and needs frequent drainage into expensive external canisters to relieve symptoms. Pleural Dynamics’ patented ACES System addresses these shortcomings2 with its one-piece, fully implanted system that is placed with a minimally invasive procedure during a short hospital stay. The device uses normal breathing motion to automatically pump pleural effusion fluid from the chest into the abdomen for reabsorption by the body, eliminating the need for an external catheter and frequent drainage. It is designed to reduce PE volume, relieve the shortness of breath associated with PE’s, and improve patient quality of life.
About Pleural Dynamics
Pleural Dynamics is a Minnesota-based medical device company, founded in 2020 by pulmonologist Dr. Martin Mayse and medtech veteran John Streeter. Frustrated with the current costly, cumbersome, and decades-old technologies to treat debilitating pleural diseases, Pleural Dynamics seeks to revolutionize care by bringing effective treatment options to patients and providers that reduce the overall cost of care through reductions in hospital stays, decreased caretaker labor and reduced infection rates. https://www.pleuraldynamics.com/
References
1 Thomas R, Fysh ETH, Smith NA, Lee P, Kwan BCH, Yap E, Horwood FC, Piccolo F, Lam DCL, Garske LA, Shrestha R, Kosky C, Read CA, Murray K, Lee YCG. Effect of an Indwelling Pleural Catheter vs Talc Pleurodesis on Hospitalization Days in Patients With Malignant Pleural Effusion: The AMPLE Randomized Clinical Trial. JAMA. 2017 Nov 21;318(19):1903-1912.
2 Heffner MD, John E. Management of Malignant Pleural Effusions. Post TW, ed. UpToDate. Waltham, MA: UpToDate Inc. http://www.uptodate.com. (Accessed on June 18, 2024).
Contacts
Martin Mayse, M.D., CEO, Pleural Dynamics, mmayse@PleuralDynamics.com