Clinical Data Presented at ACC 2025 Shows Sensydia CPS™ Provides Accurate Assessment of Mean Pulmonary Artery Pressure Non-Invasively
Clinical Data Presented at ACC 2025 Shows Sensydia CPS™ Provides Accurate Assessment of Mean Pulmonary Artery Pressure Non-Invasively
-- Non-invasive platform offers potential to transform heart failure care --
-- CPS on display for physicians in Sensydia booth #8029 at the American College of Cardiology (ACC) Annual Scientific Session & Expo --
CHICAGO--(BUSINESS WIRE)--Sensydia, a clinical-stage non-invasive cardiac assessment company, announced today that University of Minnesota investigators presented positive clinical findings from a recent study evaluating the company’s AI-powered, non-invasive Cardiac Performance System (CPS™) in a poster presentation at the American College of Cardiology (ACC) 2025 Annual Scientific Session & Expo at the McCormick Place Convention Center in Chicago, IL.
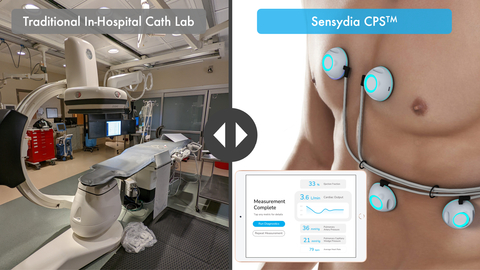
Sensydia’s CPS uses heart sound analysis to enable earlier detection and more effective therapy guidance for patients suffering from heart failure and pulmonary hypertension. To obtain these measurements today, patients must undergo echocardiography and invasive right heart catheterization, which are resource intensive, restricted to medical facilities, and only provide snapshot data. In contrast, CPS measurements are fast, safe, may be repeated as frequently as needed, and can be performed essentially anywhere with minimal training.
Poster information: |
|
Title: |
“Non-Invasive Hemodynamic Monitoring Using AI to Determine Pulmonary Artery Pressures” |
Date: |
Saturday, March 29, 2025, at 9:30-10:30 am CDT |
Location: |
Board #55 |
Key findings
- The investigator-led study enrolled 50 patients undergoing routine right heart catheterization (RHC), with CPS successfully measuring mean pulmonary artery pressure (mPAP) in 40 subjects.
- CPS demonstrated its ability to identify patients with elevated mPAP (>35mmHg and >30mmHg) with strong diagnostic accuracy, achieving an AUC of 0.80 and 0.77, respectively.
- No complications or adverse reactions were reported, and patients tolerated the non-invasive CPS measurement well.
Significance of These Findings
For clinicians and healthcare providers, these findings underscore the potential of CPS to serve as a reliable alternative to invasive RHC. CPS could provide critical, real-time insights into hemodynamics in the clinic or the patient’s home, reducing the need for hospital-based monitoring while potentially improving patient outcomes and streamlining heart failure management.
“CPS offers a fast, non-invasive, and accurate tool to assess hemodynamics that could transform heart failure care,” said Tamas Alexy, MD, PhD of the University of Minnesota. “The ability to track changes in pulmonary artery pressure (PAP) with CPS can empower clinicians to make more informed and timely treatment decisions for patients with heart failure.”
ACC.25 attendees are invited to visit booth 8029 to explore CPS’s role in improving heart failure management and see a demonstration of CPS technology.
Please note: CPS is under development and not yet FDA-approved.
About Sensydia
Sensydia is developing the Cardiac Performance System (CPS™), a non-invasive platform that provides real-time measurements of critical cardiac function. CPS is designed to deliver rapid, safe, and accurate assessments to improve outcomes for patients with heart failure and pulmonary hypertension. The company received FDA 510(k) clearance for non-invasive measurement of ejection fraction using first-generation hardware in 2018. Learn more at sensydia.com.
Contacts
Media:
Kathryn Morris, BrightPoint
kathryn@brightpointny.com
914-204-6412