TAE Life Sciences and Kyoto University Achieve Breakthrough Preclinical Results in Boron Neutron Capture Therapy (BNCT) with Promising Implications for Cancer Treatment
TAE Life Sciences and Kyoto University Achieve Breakthrough Preclinical Results in Boron Neutron Capture Therapy (BNCT) with Promising Implications for Cancer Treatment
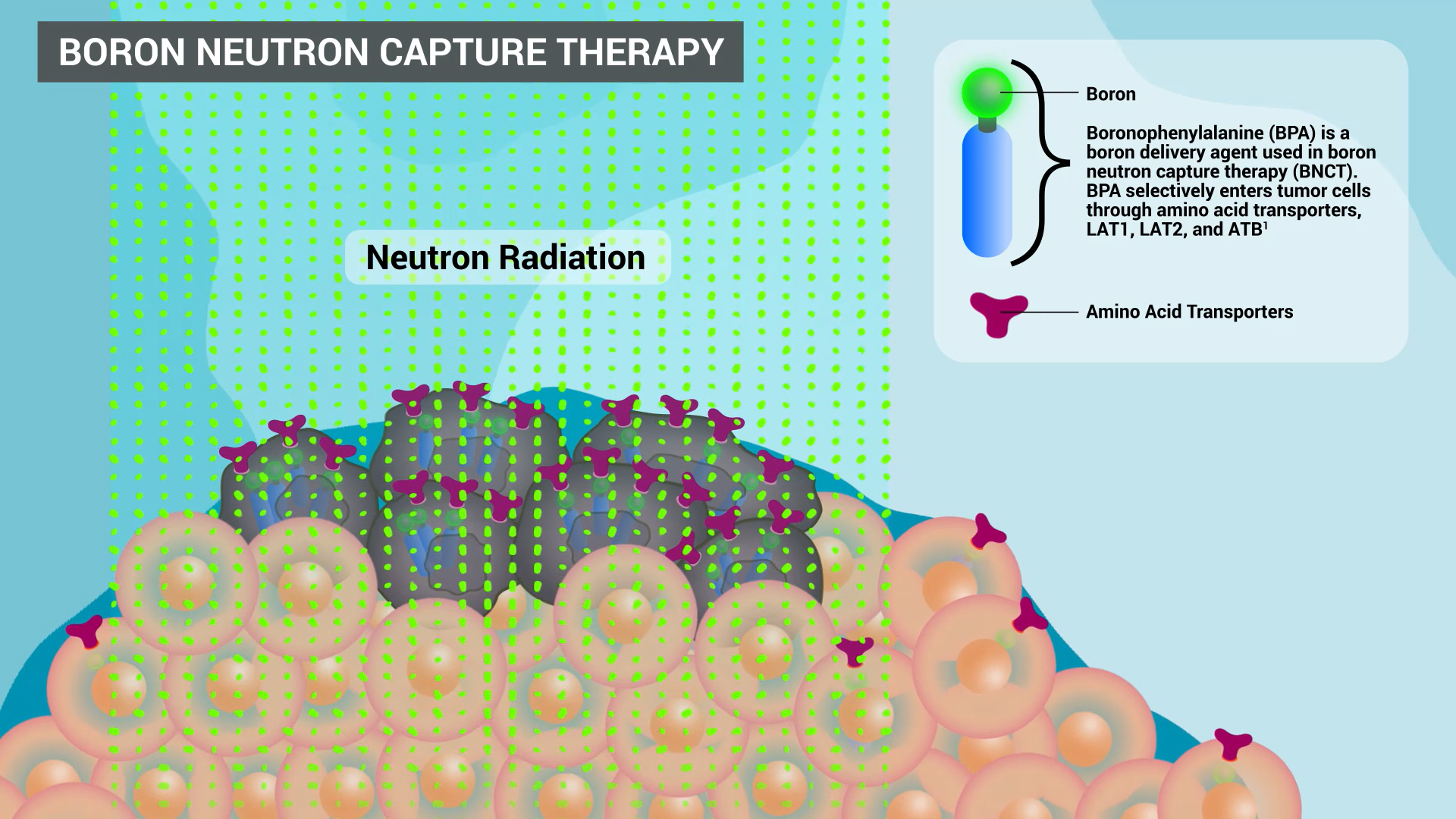
IRVINE, Calif.--(BUSINESS WIRE)--TAE Life Sciences, in collaboration with Kyoto University and Principal Investigator Dr. Fuyuhiko Tamanoi, is pleased to announce groundbreaking preclinical results in Boron Neutron Capture Therapy (BNCT). Utilizing TAE Life Science’s novel boron-10 drugs and Kyoto University Research Reactor (KURR) neutron source has generated significant pre-clinical data that may redefine the potential of BNCT in cancer treatment.
Recently published in the Journal of Medicinal Chemistry (J. Med. Chem. 2023, 66, 13809−13820) our research highlights a remarkable phenomenon known as the abscopal effect. This effect, where localized radiation treatment results in the shrinkage of tumors at untreated sites, holds profound implications for treating metastatic and micro-metastatic cancer.
In a key study using immune-competent balb/c mice, BNCT treatment of subcutaneous tumors on one leg completely inhibited tumor growth when the same tumor cells were reintroduced to the opposite leg two weeks post-treatment. These results, some of the most impressive preclinical data seen to date, suggest that BNCT can stimulate an immune response capable of generating memory cells to prevent tumor recurrence at distant sites.
In one experiment, a mouse colon tumor was grown in the leg of a mouse and a second tumor was grown in its shoulder. BNCT was applied to the tumor in the leg, while the shoulder tumor was shielded from treatment. Remarkably, the untreated shoulder tumor exhibited a 34% reduction and slower growth rate compared to the control group, highlighting a potential systemic effect of BNCT.
“While the abscopal effect is not new in radiation oncology, these results with BNCT represent a significant step forward,” said Dr. Sunil Krishnan, Radiation Oncologist and Professor at the Center for Translational Cancer Research at UT Health Houston. “What’s particularly exciting is that this level of immune response and tumor control has not traditionally been observed with standard x-ray-based radiation therapy. It underscores the potential for BNCT to not only address primary tumors but also impact metastatic disease in a way we haven’t seen before.”
Further investigations are underway to explore the mechanisms behind these results, focusing on the role of dendritic cells, T-cells, and macrophages in immune memory. Additionally, TAE Life Sciences is evaluating the combination of BNCT with immune checkpoint inhibitors, such as anti-CTLA4, anti-PD1, and anti-PDL1, to enhance outcomes in tumors with both “hot” (active immune profile) and “cold” (inactive immune profile) immune phenotypes.
"We are excited to demonstrate both the vaccine effect and the abscopal effect in preclinical BNCT experiments," said Kendall Morrison, Chief Scientific Officer at TAE Life Sciences. "This research reinforces BNCT's potential not only as a localized therapy but also as a treatment capable of addressing metastatic cancer. Combining BNCT with immunotherapy agents could significantly transform cancer treatment outcomes."
If these preclinical results can be translated to clinical applications, it could pave the way for improved responses to immune checkpoint blockade therapies, particularly for challenging “cold” tumors.
About TAE Life Sciences
TAE Life Sciences is dedicated to pioneering advancements in Boron Neutron Capture Therapy (BNCT), a unique cancer treatment modality. Through innovative compounds, cutting-edge neutron sources, and collaborations with leading institutions, TAE Life Sciences aims to offer patients a targeted, effective, and minimally invasive cancer treatment option.
Contacts
For more information, please contact:
TAE Life Sciences Media Relations
contact@taelifesciences.com
+1 949-830-2117