Novotech Releases In-Depth Report on Cervical Cancer: Addressing a Global Health Challenge
Novotech Releases In-Depth Report on Cervical Cancer: Addressing a Global Health Challenge
BOSTON--(BUSINESS WIRE)--Novotech, the global full-service clinical Contract Research Organization (CRO), has released a new report, Cervical Cancer - Global Clinical Trial Landscape. This report offers a comprehensive analysis of the global cervical cancer research landscape, highlighting the crucial role of clinical trials in advancing treatment options. It outlines key geographic trends, emerging therapies, and future projections, providing valuable insights for researchers and healthcare professionals working to combat this disease.
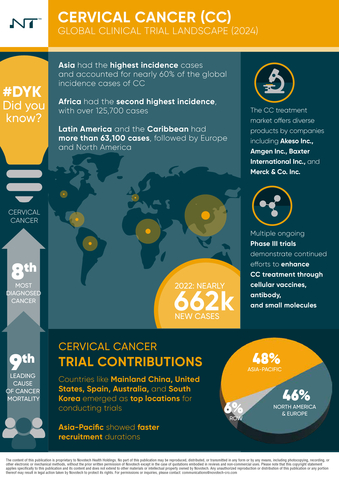
Cervical cancer continues to pose a significant global health challenge, particularly in low- and middle-income countries, with more than 662,000 new cases and 348,900 reported deaths in 2022. The World Health Organization projects a 40% rise in cases by 2050 if innovative interventions are not implemented.
Key takeaways from the report include:
- Global Burden: Cervical cancer accounted for 662,000 new cases and 348,900 deaths globally in 2022, with the highest incidence in Asia and Africa.
- Asia-Pacific Leadership: The Asia-Pacific region leads in cervical cancer clinical trials, holding 48% of the global share, with Mainland China driving much of this activity.
- Disparities in Trial Access: Despite significant trial activity in some regions, others, such as Africa, continue to face high cervical cancer incidence with limited clinical research infrastructure.
- Innovative Therapies: Emerging therapeutic strategies include immunotherapies, RNA-based treatments, and precision medicine, offering new hope for advanced cervical cancer patients.
- Biomarker Use: The increasing use of biomarkers in clinical trials helps guide personalized treatments, enhancing patient outcomes.
- AI in Research: Artificial intelligence is improving trial design, patient recruitment, and data analysis, expediting the drug development process.
- Challenges in Patient Recruitment: Geographic and logistical challenges hinder patient recruitment, particularly in low-income regions with limited access to clinical trials.
- Future Projections: Without significant intervention, cervical cancer cases are projected to increase by over 40% by 2050, reinforcing the need for global collaboration and innovative treatments.
As the global burden of cervical cancer continues to rise, the need for innovative therapies and more effective treatment strategies becomes increasingly urgent. The Novotech report highlights several key areas of opportunity for future research, including:
- Immunotherapy and RNA-based treatments: These therapies are at the forefront of cancer research and offer promising new avenues for treating cervical cancer, particularly in patients with advanced or recurrent disease.
- AI-driven clinical trials: AI technologies are revolutionizing the way clinical trials are designed, conducted, and analyzed, enabling faster and more efficient drug development processes.
- Global collaboration: International partnerships between researchers, healthcare providers, and regulatory agencies are essential to ensuring that new therapies are accessible to patients worldwide.
For a more comprehensive understanding of the current state of cervical cancer research and clinical trial development, download the full Novotech report, Cervical Cancer - Global Clinical Trial Landscape. This in-depth analysis provides valuable insights into regional trends, therapeutic innovations, and the future of cervical cancer treatment.
Download the full report here: https://novotech-cro.com/reports/cervical-cancer-global-clinical-trial-landscape-2024?utm_source=Business+Wire&utm_medium=Press+Release&utm_campaign=2024_Sep_CervicalCancer_DR_EN&utm_id=701Oc00000CfltkIAB
About Novotech Novotech-CRO.com
Founded in 1997, Novotech is a global full-service clinical Contract Research Organization (CRO) focused on partnering with biotech and small to mid-sized pharma companies to accelerate the development of advanced and novel therapeutics at every phase.
Recognized for its industry-leading contributions, Novotech has received numerous prestigious awards, including the Frost &Sullivan 2024 Global Biotech CRO Award, 2024 Employer of Choice, 2024 Great Place to Work in the US, 2024 Brandon Hall Gold Award, CRO Leadership Award 2023, the Asia Pacific Cell & Gene Therapy Clinical Trials Excellence 2023, the Asia-Pacific Contract Research Organization Company of the Year Award since 2006.
The Company offers a comprehensive suite of services including laboratories, Phase I facilities, drug development consulting, regulatory expertise, and has experience with over 5,000 clinical projects, including Phase I to Phase IV clinical trials and bioequivalence studies. With a presence in 34 office locations and a dedicated team of 3,000+ professionals worldwide, Novotech is a trusted end-to-end strategic partner of choice.
For more information or to speak to an expert team member about your clinical trial needs visit www.Novotech-CRO.com
Contacts
Media
Toyna Chin
mediacontact@novotech-cro.com
USA: +1 415 364 8135