RevBio Receives Approval to Start a Dental Clinical Trial in Italy for its Regenerative Biomaterial
RevBio Receives Approval to Start a Dental Clinical Trial in Italy for its Regenerative Biomaterial
This Study Will Help Assess the Time Necessary for Tetranite® to Regenerate Bone in the Oral Environment
LOWELL, Mass.--(BUSINESS WIRE)--RevBio, Inc., announced that it has received approval from the Italian Ministry of Health and the Ethics Committee of the University “G.D'Annunzio” of Chieti-Pescara to start a 15-patient pilot clinical trial. The primary objective of this study will be to assess the time it takes for Tetranite®, the company’s adhesive biomaterial, to regenerate bone in the mandibular and maxillary dental arches.
This study will be conducted at the Center for Advanced Studies and Technology (“CAST”) of the University “G.d’Annunzio” Chieti-Pescara under the leadership of Professor Sergio Caputi, MD, DDS, who serves as the Chancellor/Provost of the University. The research team also includes Professor Maurizio Piattelli, MD, DDS, Tonino Traini, DDS, PhD, Bruna Sinjari, DDS, PhD, Gianmaria D’Addazio, DDS, PhD, and Manlio Santilli, DDS, PhD Student. This team is part of the Department of Innovative Technologies in Medicine and Dentistry where novel and cutting-edge research in dentistry is conducted.
“We are very pleased to see this study come together after much planning during the COVID-19 pandemic,” said Dr. Sinjari. “We have assembled a great research team which will be able to assess the unique osteoconductive properties of this biomaterial.”
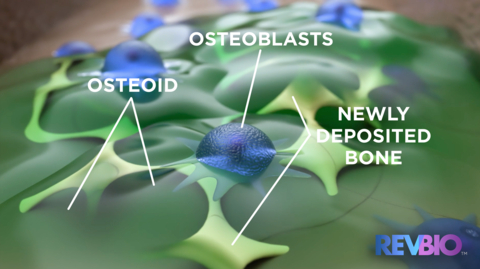
Currently there are no products approved in Europe or the United States that are adhesive to bone and capable of bonding dental implants into place while gradually resorbing and being replaced by new, native bone. These properties have been demonstrated in prior studies, but the focus of this study will be to assess the timescale when the material is resorbed by the human body, which will help determine the length of future clinical trials necessary for obtaining commercial approval.
Dr. Paul Fugazzotto, DDS, an advisor to RevBio, and a noted lecturer, dental educator, and periodontist, introduced the company to the research team at the University of Chieti. “The design of this study will answer some important questions that will help evidence the key advantages of this biomaterial,” said Dr. Fugazzotto. “I believe this technology will be transformative in the way we place dental implants.”
About RevBio, Inc.
RevBio, Inc., is a clinical stage medical device company engaged in the development and commercialization of a patented, synthetic, injectable, self-setting, and osteoconductive bone adhesive biomaterial called Tetranite®. The company is initially developing this technology for use in the dental, cranial, and broader orthopaedic markets as well as applications in the animal health market. RevBio's Tetranite technology is not yet approved for commercial use.
Contacts
Michael Tiedemann
mtiedemann@revbio.com