RevBio® Receives Approval from FDA to Start a Dental Clinical Trial for its Regenerative Bone Glue
RevBio® Receives Approval from FDA to Start a Dental Clinical Trial for its Regenerative Bone Glue
This Clinical Trial for Ridge Augmentation Procedures Expands the Portfolio of Indications for TETRANITE® Currently in Clinical Development
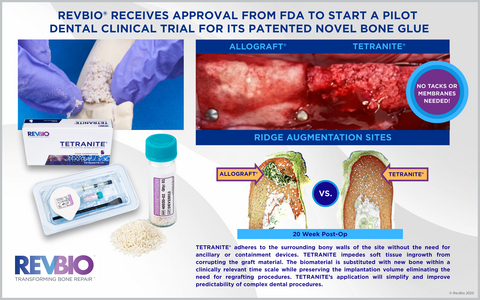
TETRANITE® adheres to the surrounding bony walls of the site without the need for ancillary or containment devices. TETRANITE impedes soft tissue ingrowth from corrupting the graft material. The biomaterial is substituted with new bone within a clinically relevant time scale while preserving the implantation volume eliminating the need for regrafting procedures. TETRANITE’s application will simplify and improve predictability of complex dental procedures.
LOWELL, Mass.--(BUSINESS WIRE)--RevBio, Inc., announced that it has received approval from the U.S. Food and Drug Administration to start a pilot clinical trial to examine the safety and efficacy of TETRANITE®, the company’s regenerative bone adhesive, to perform dental ridge augmentation procedures without the need for ancillary containment devices like membranes or meshes or fixation aids like tacks and screws.
“The ability for the product to adhere to the surrounding bony walls of a site that needs to be grafted is a fundamentally unique attribute of TETRANITE,” said Rahul Jadia, PhD, RevBio’s R&D Manager of Technology Development.
Share
“The ability for the product to adhere to the surrounding bony walls of a site that needs to be grafted is a fundamentally unique attribute of TETRANITE,” said Rahul Jadia, Ph.D., RevBio’s R&D Manager of Technology Development, who has led the development of this novel adhesive bone scaffold product. “Equally impressive is the fact that the material is substituted with bone in a clinically relevant timescale without significant loss of volume or adhesive and mechanical strength, which will ultimately help accelerate the course of complex dental procedures.”
Roughly 44% of all patients who receive a dental implant have a missing tooth when they start treatment with varying degrees of bone loss depending on how long the tooth has been missing. These patients must undergo a ridge augmentation procedure in which particulate-based bone graft materials are placed using additional fixation and containment devices to protect the graft during the healing process. These procedures increase the width and height of a jawbone to reconstruct atrophied bone so that a dental implant can be successfully placed. In over 30% of these cases, however, existing bone graft materials fail to achieve the desired clinical results, and additional bone grafting procedures must be conducted, increasing the overall time and cost of treatment.
This product development program was funded since its inception by several successive grants totaling $1.8 million from the Translational Resource Center (TRC), a research consortium (U24-DE029462) funded by the National Institute of Dental and Cranial Research (NIDCR) to improve the translation of promising tissue engineering and regenerative medicine technologies. RevBio’s funding for this program was further enhanced by a $2 million Direct to Phase II Small Business Innovation Research (SBIR) grant from NIDCR (1R44DE032564-01) to complete the pre-clinical research necessary to advance this product into its current clinical state of development.
“The TRC is excited about RevBio's unique and compelling technology that addresses a critical unmet clinical need. We are pleased to be able to support RevBio and provide the translational guidance that has helped shepherd their products to regulatory approval and embark upon first in human trials,” said David H. Kohn, Ph.D., Natalie C. Roberts Endowed Professor, Departments of Biologic and Materials Sciences and Biomedical Engineering, at the University of Michigan and Director of the Translational Resource Center.
About RevBio, Inc.
RevBio, Inc., is a clinical stage medical device company in metropolitan Boston, that has invented TETRANITE®, a regenerative bone glue currently in human patients across ten FDA approved clinical trials. Recognized as a novel technology, TETRANITE is anticipated to receive De Novo classification for several key indications which will expedite its path to commercialization. TETRANITE has demonstrated superior clinical performance over current standards of care including curing speed, shear forces, rate of bone regeneration, and wet-field adhesion. Bioengineered for a body of applications, the TETRANITE platform will address a total addressable market of over $10 billion in neurosurgical, orthopaedic and dental use cases. RevBio's TETRANITE technology is not yet approved for commercial use.
Contacts
Michael Tiedemann
mtiedemann@revbio.com