Stemson Therapeutics Announces Technological Breakthrough in New Hair Growth
Stemson Therapeutics Announces Technological Breakthrough in New Hair Growth
Demonstrates new engineered functional hair follicles in humanized mice
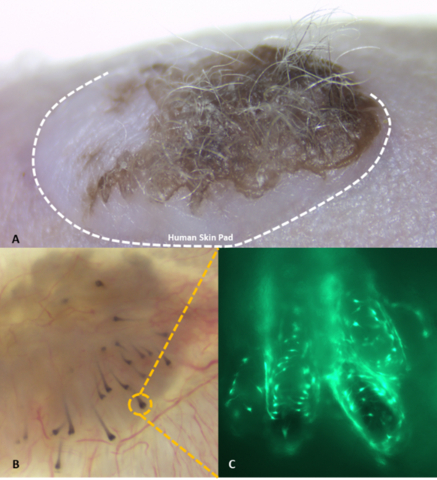
A. Hair-bearing human skin xenograft on immunocompromised nude mouse 10 weeks after transplant of Stemson engineered follicular units (EFU). B. Underside image of excised human skin xenograft showing hair bulbs generated by Stemson EFUs. C. Enlarged image of hair bulb demonstrating green fluorescent protein (GFP) labelled cells from Stemson EFUs are responsible for generating human hair follicles in skin pad. (Photo: Business Wire)
SAN DIEGO--(BUSINESS WIRE)--Stemson Therapeutics today announced a major technological advance that clears the path for its proprietary hair rejuvenation solution to advance toward human clinical trials. Stemson is developing an iPSC-derived autologous cell therapy to regenerate healthy hair follicles and has successfully created human hair follicles in humanized mice using engineered follicular units. The engineered follicular units are designed to provide an unlimited source of hair follicle replacements capable of treating a range of hair loss indications, including Androgenetic Alopecia, Scarring Alopecia, and Chemo-Induced Alopecia.
An estimated 80 million people in the United States experience hair loss, which is often associated with emotional distress leading to reduced quality of life, anxiety, and depression. Despite the $10 billion market for hair loss today, there are no existing therapeutic solutions capable of rejuvenating shrinking follicles, nor any that generate new hair follicles to replace lost hair.
“Stemson’s engineered follicular units will be the first therapeutic solution capable of replacing lost hair follicles, a breakthrough sorely needed to treat people suffering from many forms of hair loss,” says Geoff Hamilton, Chief Executive Officer of Stemson. “The recent demonstration of robust and reproducible all-human hair growth in humanized mice is a strong indicator of potential success in human trials.”
Engineered follicular units are constructed through a proprietary bioprinting process which combines hair follicle cells and biomaterials into a three dimensional biomimetic design resembling the hair follicle shape. Upon transplantation, the engineered follicular units will engraft with the host skin and develop into mature natural hair follicles replacing those that have been lost. The design of the engineered follicular units enables handling and precision delivery into the skin using common hair transplantation tools and techniques available in the field today. In addition, the design facilitates healthy follicle development with proper directionality and emergence of a hair shaft out of the skin.
To support the company’s growth and advancement toward human clinical trials, Stemson has promoted Meghan Samberg, PhD, to Chief Development Officer, and Nick Wisniewski, PhD, to Vice President of Data Science & Bioinformatics. The promotions move Stemson firmly into the final stages of product development with an emphasis on leveraging bioengineering, big data, and machine learning approaches to create a highly controlled and reproducible therapeutic outcome for hair growth.
“Stemson is poised to deliver truly novel and effective hair growth treatments to hair loss patients. Our recent results in mice engrafted with human skin show a therapeutic solution to hair loss is within our sights,” stated Dr. Samberg. “In 2019, Stemson founder Alexey Terskikh, PhD, demonstrated functional hair follicle cells grown from human stem cells can grow hair when combined with mouse cells and transplanted in mouse skin. Since then, Stemson’s development team has focused on demonstrating that an all-human bioengineered cell solution can generate brand new human hair follicles in human skin, which gives us much higher confidence the product may work in human patients. Now that we have achieved that milestone, I am excited to move us forward into the final development before testing in human trials.”
“Engineering follicular units derived from Induced Pluripotent Stem Cells is a highly promising but complex approach to solve hair loss,” added Dr. Wisniewski. “As a core part of our product development strategy at Stemson, we are investing in big data generation and cutting-edge analytics, using machine learning algorithms to manage the biological complexity and generate consistent high quality results.”
For updates on Stemson’s progress and advancement, please visit www.stemson.com.
About Stemson Therapeutics
Stemson Therapeutics is a pre-clinical stage cell therapy company founded in 2018 with a mission to cure hair loss by leveraging the regenerative power of Induced Pluripotent Stem Cells. Stemson uses iPSC to regenerate the critical cells required to grow hair and which are damaged or depleted in patients suffering from hair loss. The iPSC-derived cells are used to grow de novo hair follicles, offering a new supply of hair to treat people suffering from various forms of Alopecia. Today, there are no available treatments capable of growing new hair follicles. Stemson’s world class team of scientists, advisors and collaborators are passionate about delivering a scientifically based, clinically tested cure for hair loss to the millions of hair loss sufferers who seek help for their hair loss condition. Stemson Therapeutics is headquartered in San Diego, CA. For more information, please visit www.stemson.com.
Contacts
Amy Caterina (amy@stemsontx.com) at Stemson Therapeutics