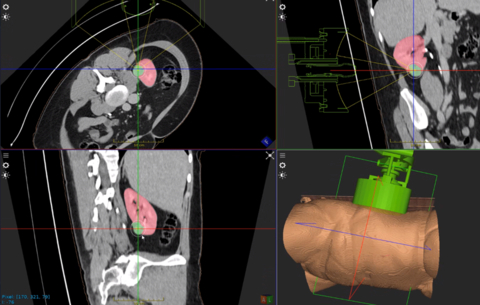
World’s First Kidney Tumor Treated Using the HistoSonics’ Edison® Histotripsy System
World’s First Kidney Tumor Treated Using the HistoSonics’ Edison® Histotripsy System
AdventHealth Celebration (FL.) Urology Performs Non-Invasive Histotripsy in Patient with Primary Solid Renal Tumor
MINNEAPOLIS--(BUSINESS WIRE)--HistoSonics, Inc., (www.histosonics.com), developer of a non-invasive platform and novel sonic beam therapy called histotripsy, announced today the treatment of the first patient in its pivotal #HOPE4KIDNEY Trial. The FDA approved the investigational device study earlier last year and it is designed to evaluate the safety and efficacy of the company’s breakthrough Edison System to destroy targeted kidney tumors, completely non-invasively and without the need for needles or incisions. The #HOPE4KIDNEY Trial is a multi-center, open label, single arm trial planned to enroll up to 68 patients.
“Our goal is to enable physicians to precisely target and destroy kidney tumors with our novel, noninvasive solution, avoiding the morbidity and complications seen with current invasive surgery or ablative techniques,” said Mike Blue, HistoSonics CEO.
Share
The procedure was performed by urologist Michael McDonald M.D., a leader and pioneer in advanced urologic procedures, including robotics, at AdventHealth Celebration in Osceola County. “At the start of my surgical career in the 1990’s open surgical techniques were the primary method of medical operations. However, this quickly changed with the introduction of laparoscopic surgery in the late 1990’s and robotic surgery in the early 2000’s,” said Dr. McDonald. “I’m excited about the potential of emerging technologies such as histotripsy to improve patient safety and outcomes.” The procedure was conducted under the auspices of the AdventHealth Research Institute, where physicians and researchers are currently conducting more than 650 studies and clinical trials. AdventHealth Celebration is internationally recognized as a hub for robotic surgery, treating patients and training surgeons from around the world in the latest techniques and technologies.
In 2020, there were an estimated 628,355 people living with kidney and renal pelvis tumors in the United States and an additional 81,000 people to be diagnosed with kidney tumors in 2023¹. Current kidney therapies such as partial nephrectomy and thermal ablation are invasive and exhibit complications from bleeding and infection that non-invasive histotripsy may avoid. While surgical intervention is the “gold standard” in removing kidney tumors, a non-invasive approach with histotripsy provides the potential to destroy targeted tumors without damaging non-targeted kidney tissue. Additionally, histotripsy’s purely mechanical mechanism of cellular destruction could preserve function of the kidney’s urine collecting system.
“We are extremely excited to have Dr. McDonald and his team at AdventHealth Celebration treat the first patient at part of the #HOPE4KIDNEY Trial,” said HistoSonics President and CEO, Mike Blue. “Our goal is to enable physicians to precisely target and destroy kidney tumors with our novel, noninvasive solution, avoiding the morbidity and complications seen with current invasive surgery or ablative techniques,” added Blue. The Company expects to take advantage of key learnings from its initial Phase I kidney study, called the CAIN Trial, and technical enhancements with its Edison platform during the #HOPE4KIDNEY Trial.
HistoSonics' image guided sonic beam therapy system uses advanced imaging and proprietary sensing technology to deliver non-invasive, personalized treatments with precision and control. The science of histotripsy uses focused sound energy to produce controlled acoustic cavitation that mechanically destroys and liquifies targeted tissue at sub-cellular levels. The company believes that the novel mechanism of action of their proprietary technology provides significant advantages to patients, including the ability of the treatment site to recover and resorb quickly. Uniquely, the HistoSonics’ platform also provides physicians the ability to monitor the destruction of tissue under continuous real-time visualization and control, unlike any modality that exists today.
The Edison® System is intended for the non-invasive mechanical destruction of liver tumors, including the partial or complete destruction of unresectable liver tumors via histotripsy. The FDA has not evaluated the Edison System for the treatment of any specific disease or condition.
Use of the Edison System in kidney applications is limited by federal law to investigational use. The #HOPE4KIDNEY Trial is expected to support a future expansion of the indication to include the destruction of kidney tissue/tumors.
¹SEER Statfacts 2020
About HistoSonics
HistoSonics is a privately held medical device company developing a non-invasive platform and proprietary sonic beam therapy utilizing the science of histotripsy, a novel mechanism of action that uses focused ultrasound to mechanically destroy and liquify unwanted tissue and tumors. The company is currently focused on the continued development of its EdisonTM Platform, global clinical studies, and new strategic projects including future clinical applications and platforms. HistoSonics has offices in Ann Arbor, Michigan and Minneapolis, MN.
For more information please visit: www.histosonics.com/
Contacts
For Media Inquiries
Media contact:
Josh King
Vice President of Marketing
Email : Joshua.king@histosonics.com
Phone : 608.332.8124