Sanguina Announces U.S. FDA-Clearance for AnemoCheck Home
Sanguina Announces U.S. FDA-Clearance for AnemoCheck Home
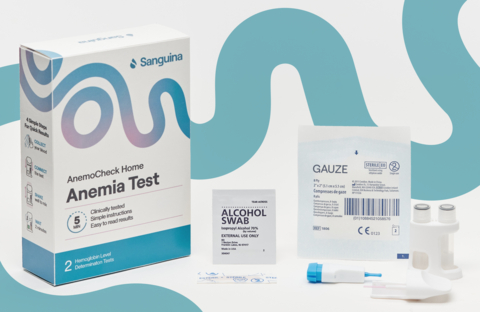
AnemoCheck Home is the only FDA-cleared home anemia test with instant results
PEACHTREE CORNERS, Ga.--(BUSINESS WIRE)--Today, Sanguina, Inc. (Sanguina), a leading biotech company, is pleased to announce the FDA clearance of AnemoCheck Home, the only FDA-cleared home hemoglobin test kit available in the United States. AnemoCheck Home is a groundbreaking test designed to empower individuals with anemia due to nutritional deficiency (iron deficiency anemia, vitamin B12 deficiency anemia, and folate deficiency anemia), sickle cell disease, and thalassemia to monitor their hemoglobin levels in the comfort of their own homes. The test is an in vitro diagnostic device and will be available by prescription only, ensuring appropriate medical oversight and guidance.
Sanguina is proud to introduce AnemoCheck Home as a game-changer for at-home anemia testing. With this FDA clearance, we are excited to provide people who have anemia with a convenient, accurate, and accessible tool.
Share
Anemia, a condition marked by a shortage of healthy red blood cells or hemoglobin, affects over 1.92 billion individuals worldwide. Acknowledging the pressing need for accessible and reliable testing, AnemoCheck Home is an innovative solution for detecting and monitoring anemia for those who need it most.
AnemoCheck Home allows users to obtain accurate hemoglobin level readings by conducting a simple fingerstick blood test. The user performs a finger stick, collects blood into a sample collection tube, and then connects the test cap with a test body. The user then shakes the test to mix the blood. After 2 minutes, the color correlates to a hemoglobin level on a color card. The test is disposable and does not require any additional equipment.
Erika Tyburski, CEO of Sanguina states: "Our team at Sanguina is proud to introduce AnemoCheck Home as a game-changer for at-home anemia testing. With this FDA clearance, we are excited to provide people who have anemia with a convenient, accurate, and accessible tool to monitor their hemoglobin levels at home.”
AnemoCheck Home leverages the latest advancements in healthcare technology, ensuring reliable and precise results to empower users to take control of their anemia management. The kit includes user-friendly instructions, and everything needed to perform the fingerstick test safely and efficiently.
To learn more about AnemoCheck Home, please visit our website at https://sanguina.com/anemocheckhome.
About Sanguina, Inc
Sanguina is a leading biotech company focused on advancing accessible digital and at-home health platforms for rapid home-based testing and disease management. Founded in 2019, Sanguina aims to empower individuals by providing innovative solutions for diagnosing, monitoring, and managing wellness and chronic conditions. We strive to bring easy-to-use and innovative medical devices and diagnostics to the fingertips of patients and healthcare professionals.
Contacts
Jessica Tackett
Director of Marketing
Sanguina Inc.
jessy.tackett@sanguina.com