Pulse Biosciences Announces Collaboration with CardioNXT for nsPFA First-in-Human Atrial Fibrillation Study
Pulse Biosciences Announces Collaboration with CardioNXT for nsPFA First-in-Human Atrial Fibrillation Study
Collaboration with CardioNXT provides navigation and mapping system integration with Pulse Biosciences’ nsPFA circumferential catheter to support first-in-human treatments
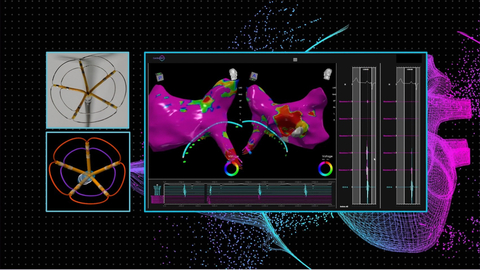
Seamless integration of Pulse Biosciences’ nsPFA cardiac circumferential catheter and CardioNXT’s 3D iMap navigation and mapping system. (1) nsPFA cardiac circumferential catheter, (2) nsPFA cardiac circumferential catheter visualized by iMap, (3) Electroanatomical map of the left atrium using the iMap system and the Pulse circumferential catheter. Courtesy of Jacob Koruth, MD, and Iwanari Kawamura, MD.
HAYWARD, Calif.--(BUSINESS WIRE)--Pulse Biosciences, Inc. (Nasdaq: PLSE, “the Company”), a company primarily focused on leveraging its novel and proprietary Nanosecond Pulsed Field Ablation (nsPFA) technology for the treatment of atrial fibrillation, today announced a collaboration with CardioNXT, to support Pulse Biosciences’ planned nsPFA cardiac catheter first-in-human study focused on the treatment of atrial fibrillation.
“We are excited to announce this collaboration and the progress we’ve made integrating our circumferential nsPFA catheter with the CardioNXT iMap System,” said Kevin Danahy, Chief Executive Officer of Pulse Biosciences. “The CardioNXT navigation and mapping system was our top choice for integration with our novel nsPFA cardiac circumferential catheter. Their iMap system offers dynamic referencing to provide superior accuracy and stability when navigating our catheter to targeted tissue areas. We’re on track to initiate our first-in-human study in the first half of next year and believe this combination has the potential to achieve best-in-class clinical outcomes.”
The collaboration will include full integration of Pulse Biosciences’ nsPFA circumferential cardiac catheter and CardioNXT’s iMap Navigation and Mapping System, enabling electrophysiologists to successfully visualize individual cardiac structures and place the nsPFA catheter for circumferential ablations of targeted pulmonary veins in the treatment of atrial fibrillation.
“We are excited to partner with Pulse Biosciences to advance our shared mission of improving cardiac therapy while further demonstrating the versatility of iMap. Pulse’s nsPFA technology provides a highly differentiated catheter-based solution and the integration with our system has the potential to provide fast, accurate, effective, and safe zero-fluoro ablation of cardiac tissue for the treatment of AF,” said Jerome Edwards, Chief Executive Officer of CardioNXT.
About Pulse Biosciences®
Pulse Biosciences is a novel bioelectric medicine company committed to health innovation that has the potential to improve the quality of life for patients. The Company’s proprietary Nanosecond Pulsed Field Ablation (nsPFA) technology delivers nanosecond pulses of electrical energy to non-thermally clear cells while sparing adjacent noncellular tissue. The Company is actively pursuing the development of its nsPFA technology for use in the treatment of atrial fibrillation and in a select few other markets where nsPFA could have a profound positive impact on healthcare for both patients and providers.
Pulse Biosciences, CellFX, Nano-Pulse Stimulation, NPS, nsPFA and the stylized logos are among the trademarks and/or registered trademarks of Pulse Biosciences, Inc. in the United States and other countries.
About CardioNXT, Inc.
CardioNXT has developed a commercially available 3D Mapping & Navigation System that leverages internal navigation referencing with electromagnetic tracking and AI enabled complex mapping algorithms. The CardioNXT Team has a track record of creating market leading 3D Navigation & Mapping products such as the Medtronic-StealthStation, Medtronic-LocaLisa, and Olympus-Veran Systems which have enjoyed market leadership positions in multiple clinical applications.
Contacts
Pulse Biosciences
Kevin Danahy, President and CEO
510.241.1077
IR@pulsebiosciences.com
or
Gilmartin Group
Philip Trip Taylor
415.937.5406
philip@gilmartinir.com