Organ Recovery Systems’ LifePort® Liver Transporter Preserves First Livers for Transplant as Part of the PILOT™ Continued Access Study
Organ Recovery Systems’ LifePort® Liver Transporter Preserves First Livers for Transplant as Part of the PILOT™ Continued Access Study
When used to preserve donor livers from extended criteria donors, newly reported clinical trial outcomes show LifePort Liver Transporter improves graft and patient survival compared to static cold storage
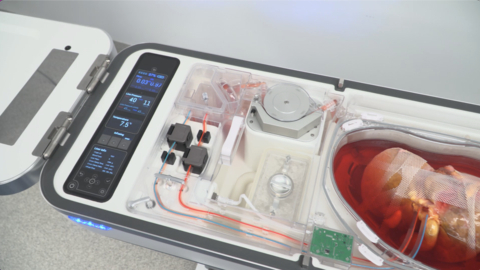
ITASCA, Ill.--(BUSINESS WIRE)--Organ Recovery Systems (ORS) is celebrating the first liver transplants using the LifePort Liver Transporter (LLT) with Vasosol® at Rutgers Medical School in New Jersey and Northwestern Memorial Hospital in Chicago, Illinois. LLT uses oxygenated hypothermic machine perfusion (HMP) to limit organ damage during preservation, resulting in improved patient outcomes and increased utilization of lifesaving organs. This milestone took place as part of the FDA-authorized Continued Access study confirming the findings of the PILOT pivotal efficacy trial conducted over the last two years under a US FDA Investigational Device Exemption.
Currently, around 11,000 Americans are waiting to receive a liver transplant. The traditional preservation method for donor organs is static cold storage, which limits the time and distance an organ can travel to the recipient transplant center. In contrast, LLT continuously perfuses a donor liver with a physiologic, oxygenated, nutrient-rich cold solution that keeps the organ healthy during preservation, potentially enabling donations over longer distances and extending the time the organ is viable for transplantation. Results of the multi-center PILOT trial presented at the 2022 American Association for the Study of Liver Disease (AASLD) Industry Colloquium showed a decreased length of patient hospital stay and reduced medical complications, leading to an improvement in graft function and patient survival.
“Organ transplant involves a diverse team of people who are passionate about healthcare yet often find they must rush to deliver organs during their limited viability window. And that’s typically the case, even with the healthiest of donor organs,” said David Kravitz, CEO of ORS. “From the promising clinical evidence we’ve seen thus far, use of LLT makes the process easier on everyone involved, which gives patients on the waiting list a better chance of receiving a second chance at life.”
The first livers preserved with the LLT under Continued Access were transplanted at University Hospital in Newark, NJ. Rutgers New Jersey Medical School is the first of a larger effort to further investigate the benefits of integrating LLT into existing transplant programs at participating medical centers.
"I am extremely excited to finally be able to use oxygenated HMP for my patients as a targeted intervention to improve logistics, outcomes, and access to transplant by safely facilitating the use of Extended Criteria and DCD livers,” said Dr. James V. Guarrera, Professor of Surgery and Chief of Transplant and HPB Surgery at Rutgers New Jersey Medical School and Program Director at UH Liver Transplant.
ABOUT ORGAN RECOVERY SYSTEMS
Organ Recovery Systems is a global market-leading provider of organ preservation products and services, supporting 320 transplant programs across 43 countries. Since its launch in 2003, LifePort Kidney Transporter has been employed in over 175,000 renal transplant procedures worldwide. Organ Recovery Systems’ proprietary LifePort platform has also been successfully designed to accommodate donor livers to help improve transplant outcomes for end-stage liver disease patients. LifePort Kidney and Liver Transporters are among a growing family of products and services developed by Organ Recovery Systems to support the Company’s mission of honoring donation as a gift of life, improving outcomes, and helping lower overall costs of care for end-stage organ disease.
Contacts
CG Life
Sarah Mishek
SMishek@CGLife.com