Cutera Announces Over 1,000 AviClear™ Acne Treatments Performed
Cutera Announces Over 1,000 AviClear™ Acne Treatments Performed
AviClear becomes the fastest adopted device in Cutera’s history
BRISBANE, Calif.--(BUSINESS WIRE)--Cutera, Inc. (Nasdaq: CUTR) ("Cutera" or the "Company"), a leading provider of aesthetic and dermatology solutions, today announces that over 1,000 AviClear laser treatments have been performed in the United States. AviClear is the first and only energy-based device to be FDA-cleared for the treatment of mild, moderate and severe acne. Additionally, AviClear recently received approval from Health Canada for the treatment of all severities of acne as well as the treatment of acne scars.
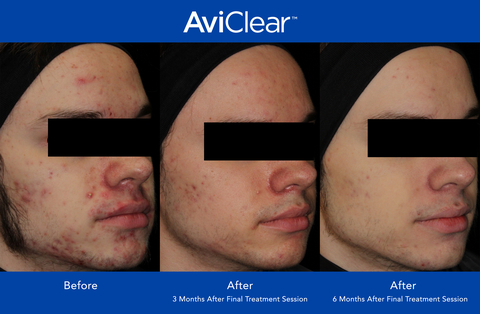
AviClear has seen growing physician adoption and steadily increasing patient demand since its launch in April 2022. Through the application of the company’s proprietary technologies, AviClear significantly reduces the severity and frequency of acne, with 90% of patients showing visible improvement at six months after completion of the treatment protocol.1 AviClear utilizes a first-to-market 1726nm wavelength laser to treat acne at the source, selectively targeting and suppressing the sebaceous glands. Treatment satisfaction ratings are high, with patients rating their AviClear treatment 4.9 out of 5 stars.2
“AviClear is the treatment my acne patients have been waiting for,” said Michael H. Gold, MD, Founder of Gold Skin Care Center. “It’s a game-changer for physicians helping to tackle the challenge of compliance while offering an effective solution for those who have not wanted or could not proceed with prescription options like antibiotics or isotretinoin for their acne. The treatment is also well tolerated, and no pain mitigation is needed with the integrated AviCool™ contact cooling system providing comfort and safety. Because it can be used on all skin types and acne severities without adverse effects, AviClear is bringing new patients to my practice — people who thought they had no other options to help their acne.”
“We continue to be excited with the clinical results our physician partners are achieving with the AviClear device,” said David Mowry, CEO of Cutera. “We’ve been exceptionally pleased with the speed of integration of this novel device into dermatology practices across the country. Additionally, the amount of outreach from patients looking to learn about this breakthrough treatment has far exceeded any other launch in our company’s history.”
“Physicians and patients alike are quickly realizing the effectiveness of the AviClear treatment as well as the unmet need AviClear fills in the modern skincare market,” said Mowry. “Positive results, ease of use, and our specialized partnership program, Avi360, have made it possible to deliver a patient-and-practice-friendly treatment option while alleviating the administrative burdens attributed to modern acne care.”
AviClear remains in a limited commercial release to physicians across the United States and was recently introduced in Canada. Doctors and patients are encouraged to visit www.AviClear.com to sign up for updates on product availability and local treatment providers.
About Cutera, Inc.
Brisbane, California-based Cutera is a leading provider of aesthetic and dermatology solutions for practitioners worldwide. Since 1998, Cutera has been developing innovative, easy-to-use products that harness the power of science and nature to enable medical practitioners to offer safe and effective treatments to their patients. For more information, call +1-415-657-5500 or 1-888-4CUTERA or visit www.cutera.com.
References:
- Data on file. FDA clearance study. Cutera, Inc.
- Data on file. Cutera, Inc.