Advanced Chemotherapy Technologies, Inc. Awarded $4 Million NIH Grant to Pursue Treatment for Locally Advanced Non-resectable Pancreatic Cancer
Advanced Chemotherapy Technologies, Inc. Awarded $4 Million NIH Grant to Pursue Treatment for Locally Advanced Non-resectable Pancreatic Cancer
This Grant will fund completion of the Company’s Pre-Clinical Research and First in Human Phase 1B Clinical Study
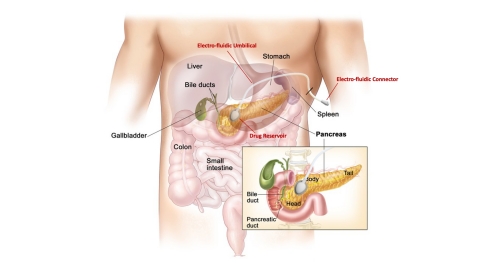
RALEIGH, N.C.--(BUSINESS WIRE)--Advanced Chemotherapy Technologies (ACT), Inc., a clinical-stage drug delivery company, announced today that it has been awarded a Phase IIb Small Business Innovation research (SBIR) grant expected to total $4 million over two years from the National Cancer Institute, part of the National Institutes of Health. The grant, in combination with prior financing, supports development of the company’s ACT-IOP-003 local drug delivery system for the treatment of locally advanced non-resectable and borderline resectable pancreatic cancer.
Pancreatic cancer is a devastating disease with 5-year survival rates of only 10%. New treatment modalities are desperately needed and ACT is developing the ACT-IOP-003 system for the targeted delivery of the FDA-approved chemotherapy treatment gemcitabine. ACT-IOP-003 enables the delivery of significantly higher concentrations directly to the tumor than achievable in systemic delivery, while also minimizing the systemic exposure and toxicity. This approach offers three major advantages over traditional systemic chemotherapy: (1) superior delivery of chemotherapy to the targeted tumor cells that are often shrouded in stroma within growing tumors, greatly increasing the amount of drug to treat the growing tumor, (2) tumor shrinkage that can enable surgical resection, the only curative treatment for pancreatic cancer, and (3) greatly decreased systemic toxicity so that the patient can better tolerate conventional approaches to their treatment.
William Daunch PhD., ACT’s Chief Technology Officer will serve as the Principal Investigator on the grant. Dr. Daunch says, “We are thrilled and encouraged that NCI has chosen to award us this highly competitive Phase IIb grant. It demonstrates the NIH’s positive recognition of the work we completed in our earlier phases, as well as their continued confidence in our program to accelerate this potentially life extending treatment to patients.”
NIH sponsored grant programs are an integral source of capital for early-stage U.S. small businesses that are creating innovative technologies to improve human health. These programs help small businesses break into the federal research and development arena, create life-saving technologies, and stimulate economic growth. ACT is honored to be a recipient of this competitive award from the NIH/NCI and looks forward to advancing treatment for pancreatic cancer patients and expanding its local drug delivery technology in new indications.
About Advanced Chemotherapy Technologies
Advanced Chemotherapy Technologies, Inc. is a privately owned, clinical-stage company developing novel approaches to local drug delivery. Its lead product is the first-in-class to combine iontophoresis drug delivery with an implantable delivery system to target drug delivery with laser-like precision. Advanced Chemotherapy Technologies drug delivery technology was first developed at and licensed from the University of North Carolina at Chapel Hill in conjunction with UNC Lineberger Comprehensive Cancer Center from the laboratories of Dr. Jen Jen Yeh, Oliver Smithies Investigator; Professor, Departments of Surgery and Pharmacology; Vice Chair for Research, Department of Surgery; Co-Associate Director of Education, Lineberger Comprehensive Cancer Center; Co-Program Leader, Clinical Translational Research Program, Lineberger Comprehensive Cancer Center; Co-Director Pancreatic Cancer Center of Excellence, Lineberger Comprehensive Cancer Center, and Joseph M. DeSimone, Ph.D, the Sanjiv Sam Gambhir of Translational Medicine and Chemical Engineering at Stanford University and Professor Emeritus, University of North Carolina at Chapel Hill and NC State.
Advanced Chemotherapy Technologies has received funding from the U.S. National Institutes of Health (NIH) through SBIR awards, the North Carolina Biotechnology Center through strategic loans, and prior investment rounds. The initial development and preclinical studies of the technology, which took place at the University of North Carolina, were funded through grants from the University Cancer Research Fund, the NIH, and the NIH Director’s Pioneer Award Program.
Contacts
Tony Voiers, CEO
(919)917-7324
info@advancedchemotech.com
www.advancedchemotech.com
https://twitter.com/advchemotech