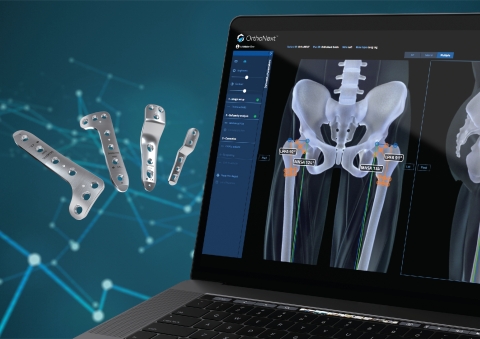
LEWISVILLE, Texas--(BUSINESS WIRE)--Orthofix Medical Inc. (NASDAQ:OFIX), a global medical device company with a spine and orthopedics focus, today announced expansion of its pediatric offerings with the U.S. Food and Drug Administration (FDA) 510(k) clearance of the OrthoNext™ digital platform, the only software tool in the market for deformity analysis and preoperative planning for pediatric orthopedic procedures.
Developed specifically for use with the JuniOrtho Plating System™, the OrthoNext digital platform software enables the surgeon to accurately plan the osteotomy position to visualize the implant in relation to the anatomy. This unique platform is designed to streamline the selection of the precise size of device and enable optimal positioning for the patient’s body prior to the surgical procedure.
With this clearance, Orthofix has also initiated its U.S. and European full market launch of the JuniOrtho Plating System. Created specifically for pediatric patients, the JuniOrtho Plating System is designed to address the demands of advanced deformity and trauma reconstruction of the lower extremities.
“Together, OrthoNext and the JuniOrtho Plating System provide a complete solution for surgeons looking for a plating system to address the specific demands of advanced deformity and trauma reconstruction of the lower extremities in the pediatric population,” said Orthofix President of Global Orthopedics Paul Gonsalves. “We are very pleased to receive the FDA clearance of the OrthoNext platform and excited to move forward with our full market launch of the JuniOrtho Plating System as we continue to deliver on our commitment to advancing pediatric orthopedics.”
The JuniOrtho Plating System is offered in a wide range of plate sizes with a variety of lengths and accommodates both locking and non-locking screws corresponding to the plate size. The system is comprised of sterile implants and single-use tools to optimize efficiency during the procedure.
The OrthoNext platform and the JuniOrtho Plating System will be on display at the Pediatric Orthopaedic Society of North America (POSNA) annual meeting in Dallas, Texas May 12-15. For those attending POSNA, please visit booth 13 to learn more about Orthofix’s JuniOrtho line of pediatric solutions that includes the TL-HEX TrueLok Hexapod System™, TrueLok™ Ring Fixation System, eight-Plate Guided Growth System+, and many others. JuniOrtho brings products and resources together to give both medical professionals and families the best in pediatric orthopedic solutions. To learn more, please visit www.juniortho.club.
About Orthofix
Orthofix Medical Inc. is a global medical device and biologics company with a spine and orthopedics focus. The Company’s mission is to deliver innovative, quality-driven solutions as we partner with health care professionals to improve patient mobility. Headquartered in Lewisville, Texas, Orthofix’s spine and orthopedic products are distributed in more than 70 countries via the Company's sales representatives and distributors. For more information, please visit www.Orthofix.com.
Forward-Looking Statements
This communication contains forward-looking statements within the meaning of Section 21E of the Securities Exchange Act of 1934, as amended, and Section 27A of the Securities Act of 1933, as amended, relating to our business and financial outlook, which are based on our current beliefs, assumptions, expectations, estimates, forecasts and projections. In some cases, you can identify forward-looking statements by terminology such as “may,” “will,” “should,” “expects,” “plans,” “anticipates,” “believes,” “estimates,” “projects,” “intends,” “predicts,” “potential,” or “continue” or other comparable terminology. These forward-looking statements are not guarantees of our future performance and involve risks, uncertainties, estimates and assumptions that are difficult to predict, including the risks described in Part I, Item 1A under the heading Risk Factors in our Annual Report on Form 10-K for the year ended December 31, 2020 (the “2020 Form 10-K”). In addition to the risks described there, factors that could cause or contribute to such differences may include, but are not limited to; the risk that surgeons may be slow to adopt the OrthoNext digital platform; the risk that future patient studies or clinical experience and data may indicate that treatment with the OrthoNext digital platform does not improve patient outcomes as much as previously believed, or otherwise call into question the benefits of its use to patients, hospitals and surgeons; the risk that the product may not perform as intended and may therefore not achieve commercial success; the risk that competitors may develop superior products or may have a greater market position enabling more successful commercialization; the risk that insurance payers may decline to reimburse healthcare providers for the use of our products.
This list of risks, uncertainties and other factors is not complete. We discuss some of these matters more fully, as well as certain risk factors that could affect our business, financial condition, results of operations, and prospects, in reports we file from time-to-time with the SEC, which are available to read at www.sec.gov. Any or all forward-looking statements that we make may turn out to be wrong (due to inaccurate assumptions that we make or otherwise), and our actual outcomes and results may differ materially from those expressed in these forward-looking statements. You should not place undue reliance on any of these forward-looking statements. Further, any forward-looking statement speaks only as of the date hereof, unless it is specifically otherwise stated to be made as of a different date. We undertake no obligation to update, and expressly disclaim any duty to update, our forward-looking statements, whether as a result of circumstances or events that arise after the date hereof, new information, or otherwise.