Doctor Evidence Introduces DOC Search COVID-19 Digest
Doctor Evidence Introduces DOC Search COVID-19 Digest
Monthly overview will highlight vast amount of information being released about COVID-19
SANTA MONICA, Calif.--(BUSINESS WIRE)--Doctor Evidence is launching a monthly DOC Search COVID-19 Digest to inform the public about the amount of information being released that covers COVID-19. The vast and ever-increasing stream of information related to the disease is difficult to harness and understand. Doctor Evidence is introducing this digest as part of our mission to use intelligent, cutting edge technology and expertise to deliver evidence-based answers and meaningful healthcare insights.
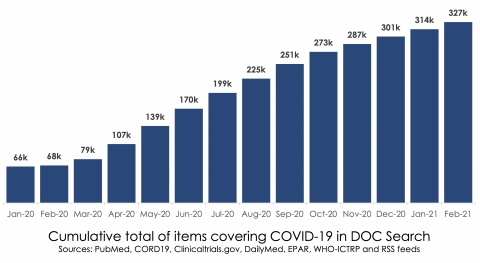
The month of February 2021 saw 327,000 total items published relating to COVID-19, approximately 5 times the volume seen in January 2020.
“COVID-19 continues to dramatically impact our daily lives, and with the global health community discovering new variants, a significant amount of research continues being conducted, reviewed and discussed,” stated Bob Battista, CEO, Doctor Evidence. “We are also pleased to be supporting The Trinity Challenge and teams submitting challenges to provide insight into the tremendous volume of medical information being released.”
DOC Search is a specialized medical search engine that generates insights and answers critical business and research questions in real time based on the universe of published medical information. Based on advanced artificial intelligence, DOC Search exhaustively searches all of the global literature derived from continuous indexing of PubMed, ClinicalTrials.gov, DailyMed, EPAR, WHO-ICTRP and individually selected global health-related RSS feeds. It currently contains more than 34.8 million items, generating 3.1 million medical concepts and 4.6 million terms. COVID-19-related items are a small part of the universe of medical literature contained in DOC Search.
About Doctor Evidence:
Doctor Evidence is the leading medical intelligence platform for Life Sciences companies that enables users to identify breakthrough insights grounded in the vast universe of published medical information, real-world evidence and proprietary data. It pushes the boundaries of healthcare technology and allows for new possibilities in science, enabling more informed decision making and faster time-to-market for accelerated impact.
Contacts
Cathy Finley
+1 (310) 595-1265
pr@doctorevidence.com
https://drevidence.com/