Verve Therapeutics Announces Data Demonstrating Durable LDL Cholesterol Lowering After a One-Time Gene Editing Treatment in Non-Human Primates
Verve Therapeutics Announces Data Demonstrating Durable LDL Cholesterol Lowering After a One-Time Gene Editing Treatment in Non-Human Primates
Results Validate In Vivo Liver DNA Base Editing as a Once-and-Done Approach to Treat Coronary Heart Disease by Lowering of LDL Cholesterol and PCSK9 Protein Levels
Company Selects VERVE-101 as Lead Program to Treat Heterozygous Familial Hypercholesterolemia
CAMBRIDGE, Mass.--(BUSINESS WIRE)--Verve Therapeutics, a biotech company pioneering gene editing medicines to treat cardiovascular disease, today announced new data demonstrating both durable and consistent lowering of blood low-density lipoprotein cholesterol (LDL-C) levels at six months after a single gene editing treatment in non-human primates. Additionally, the company has selected VERVE-101 as its lead product to be developed initially for the treatment of heterozygous familial hypercholesterolemia (HeFH), a potentially fatal genetic heart disease. VERVE-101 consists of an adenine base editor messenger RNA and an optimized guide RNA targeting the PCSK9 gene packaged in an engineered lipid nanoparticle.
HeFH causes lifelong, high LDL-C levels that leads to early coronary heart disease and affects approximately 1 in 200 to 500 individuals worldwide.1 Individuals with HeFH have a genetic mutation, typically in the LDL receptor (LDLR) gene, which down-regulates LDLR expression, causing extremely high LDL-C levels in the blood. Over time, high LDL-C builds up in the heart’s arteries, resulting in reduced blood flow or blockage, and ultimately heart attack or stroke. Inactivation of the PCSK9 gene has been shown to up-regulate LDLR expression, which leads to lower LDL-C levels, thereby reducing the risk for coronary heart disease.2 By making a single A-to-G change in the DNA genetic sequence of PCSK9, VERVE-101 aims to inactivate the target gene.
“Coronary heart disease, the leading cause of death worldwide, is driven by cumulative exposure to LDL-C, and yet, current treatment approaches fail to sufficiently and durably reduce that exposure. As a result, many patients, particularly those with HeFH, suffer more heart attacks,” said Andrew Bellinger, M.D., Ph.D., chief scientific officer of Verve. “These exciting results show, for the first time, the durable and sustained effects of a single dose of our gene editing medicine, validating this approach as a potential long-term, once-and-done treatment for coronary heart disease. Based on these and other preclinical data, we have initiated IND-enabling studies for VERVE-101 and are on track to begin clinical development in 2022.”
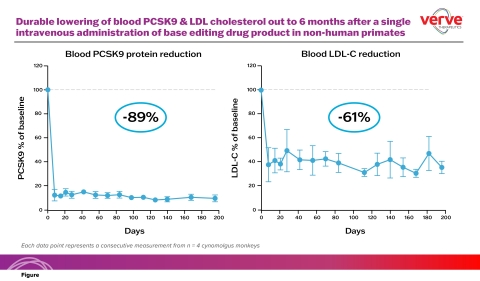
The new data demonstrate that in vivo base editing of the PCSK9 gene in the liver of non-human primates resulted in durable and consistent lowering of blood LDL-C and blood PCSK9 protein levels following a single course of treatment. In the studies, a single intravenous infusion achieved a 59% reduction in blood LDL-C at two weeks, which has been maintained at six months post treatment. LDL-C reduction over this time period averaged 61% (see Figure). During this same six-month time period, the average blood PCSK9 protein level was reduced by 89%.
In addition, Verve’s gene editing treatment was well tolerated with no adverse events reported during the study. In studies of primary human hepatocytes, clear evidence of on-target editing was observed with no evidence of off-target editing.
“Our goal is to develop gene editing medicines that can transform the treatment of cardiovascular disease from chronic care to a once-and-done treatment,” said Sekar Kathiresan, M.D., chief executive officer of Verve. “We’ve made significant progress across our pipeline, generating robust preclinical safety and efficacy data in non-human primates, and the selection of the first development candidate from our pipeline of gene editing medicines is a meaningful milestone for Verve. With these data, we are a significant step closer to delivering on our vision of providing a single-course treatment to treat, and ultimately eradicate coronary heart disease.”
Base editing, which Verve has exclusively licensed from Beam Therapeutics for certain cardiovascular targets, is a gene editing technology developed to enable precise and permanent rewriting of a single DNA letter in the genome.
About VERVE-101
VERVE-101, Verve’s lead product candidate, is being developed for the treatment of adults with severe HeFH, a potentially fatal inherited disease that causes high levels of LDL-C and early coronary heart disease. VERVE-101 is designed to be a one-time treatment, delivered via intravenous infusion, that uses adenine base editing to safely and precisely turn off PCSK9 in the liver and permanently lower LDL-C levels to treat coronary heart disease. By making a single, precise A-to-G spelling change in the DNA genetic sequence of the PCSK9 gene in liver, VERVE-101 aims to inactivate the target gene.
About Verve Therapeutics
Verve Therapeutics is a biotechnology company created with a singular focus: to protect the world from heart disease. The company brings together human genetics analysis and gene editing – two of the biggest breakthroughs in 21st century biomedicine – to develop transformative therapies for coronary heart disease. Verve is developing medicines, administered once in life, to safely edit the genome of adults and mimic naturally occurring gene variants to permanently lower LDL cholesterol and triglyceride levels and thereby treat coronary heart disease. Founded by world-leading experts in cardiovascular medicine, human genetics and gene editing, Verve is backed by a top-tier syndicate of investors, including GV (formerly Google Ventures), ARCH Venture Partners, F-Prime Capital, Biomatics Capital, Wellington Management, Casdin Capital, and Partners Innovation Fund. Verve is headquartered in Cambridge, Massachusetts. For more information, visit www.VerveTx.com.
References
1. Nordestgaard BG et al. Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: guidance for clinicians to prevent coronary heart disease: consensus statement of the European Atherosclerosis Society. Eur Heart J. 2013;34(45):3478-90a.
2. Mach F et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41(1):111-188.
Contacts
Media Contact
Gina Nugent, 617-460-3579
Ten Bridge Communications
gina@tenbridgecommunications.com
Investor Contact
Monique Allaire
THRUST Strategic Communications
monique@thrustsc.com