Orthofix FIREBIRD SI Fusion System Receives Additional FDA Clearance for Nanotechnology
Orthofix FIREBIRD SI Fusion System Receives Additional FDA Clearance for Nanotechnology
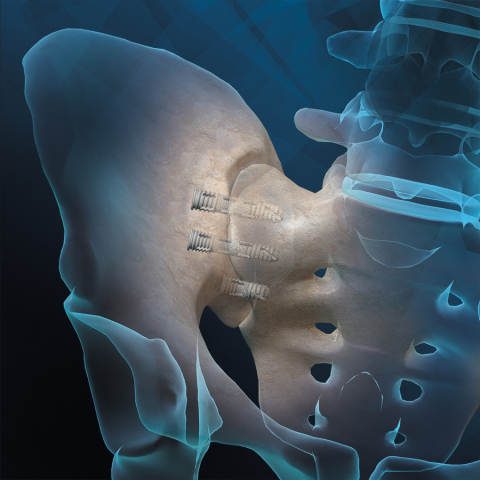
LEWISVILLE, Texas--(BUSINESS WIRE)--Orthofix Medical Inc. (NASDAQ:OFIX), a global medical device and biologics company with a spine and extremities focus, today announced the U.S. Food and Drug Administration (FDA) 510(k) clearance for the nanotechnology feature of the FIREBIRD™ SI Fusion System. Introduced earlier this year, the FIREBIRD SI Fusion System is the first 3D-printed titanium bone screw with nanotechnology specifically designed to compress and stabilize the sacroiliac joint (SI joint) during fusion.
“The FIREBIRD SI Fusion System with NANOVATE™ technology is one of the many new differentiated solutions that Orthofix is proud to highlight during the upcoming North American Spine Society (NASS) 2020 virtual annual meeting,” said Kevin Kenny, Global President of Orthofix Spine. “The clearance of the nanotechnology feature gives us the opportunity to educate surgeons about the unique benefits of the system’s nano-surface. Created through a proprietary manufacturing process, the FIREBIRD SI Fusion System is the result of our intense focus on bringing innovations to the market to enable surgeons to meet the needs of their patients.”
The FIREBIRD SI Fusion System is implanted through a minimally invasive procedure that involves inserting two to four bone screws across the SI joint to stabilize the joint during the fusion process. The system’s 3D-printed mid-shaft porous region is designed to allow for bone through growth through the device to aid in the fusion process for patients being treated for pain and dysfunction of the SI joint.”
Featuring a cannulated screw design, the system enables surgeons to pack the device with autograft and/or allografts to help promote bone fusion. The FIREBIRD SI screws are available in an assortment of lengths and diameters to address a variety of patient anatomies. Orthofix offers allograft solutions such as the Trinity ELITE™ allograft with viable cells through a partnership with MTF Biologics.
Common causes that may lead to SI joint dysfunction and pain include trauma, lifting or twisting, pregnancy, natural childbirth, degeneration from previous lumbar spine surgery, stresses to the joint due to leg length differences, joint replacement or scoliosis among others. Published clinical literature indicates that sacroiliac joint pain is estimated to affect between 15 and 30 percent of individuals with chronic low back pain.
About Orthofix
Orthofix Medical Inc. is a global medical device and biologics company with a spine and extremities focus. The Company’s mission is to deliver innovative, quality-driven solutions as we partner with health care professionals on improving patients’ lives. Headquartered in Lewisville, Texas, Orthofix’s products are distributed in more than 70 countries via the Company’s sales representatives and distributors. For more information, please visit www.Orthofix.com.
Forward Looking Statements
This communication contains certain forward-looking statements under the Private Securities Litigation Reform Act of 1995. These forward-looking statements, which may include, but are not limited to, statements concerning the estimates, projections, financial condition, results of operations and businesses of Orthofix and its subsidiaries, are based on Orthofix management's current expectations and estimates and involve risks and uncertainties that could cause actual results or outcomes to differ materially from those contemplated by the forward-looking statements.
The forward-looking statements in this release do not constitute guarantees or promises of future performance. Factors that could cause or contribute to such differences may include, but are not limited to, risks relating to: practices of health insurance companies and other third-party payors with respect to reimbursement for our devices and other risks described in the "Risk Factors" sections of our 2019 Annual Report on Form 10-K and our Quarterly Reports on Form 10-Q for the periods ended March 31, 2020 and June 30, 2020. Existing and prospective investors are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. The Company undertakes no obligation to update or revise the information contained in this press release.
Contacts
Mark Quick
Investor Relations
Tel 214 937 2924
markquick@orthofix.com
Denise Landry
Media Relations
Tel 214 937 2529
deniselandry@orthofix.com