NovaBay Pharmaceuticals Signs Agreement to Distribute COVID-19 Antibody Rapid Point-of-Care Test to U.S. Healthcare Professionals
NovaBay Pharmaceuticals Signs Agreement to Distribute COVID-19 Antibody Rapid Point-of-Care Test to U.S. Healthcare Professionals
Company to submit the ISO 13485 and CE Mark certified fluorecare® test kit under FDA’s EAU and 510(k) clearance for U.S. commercial use
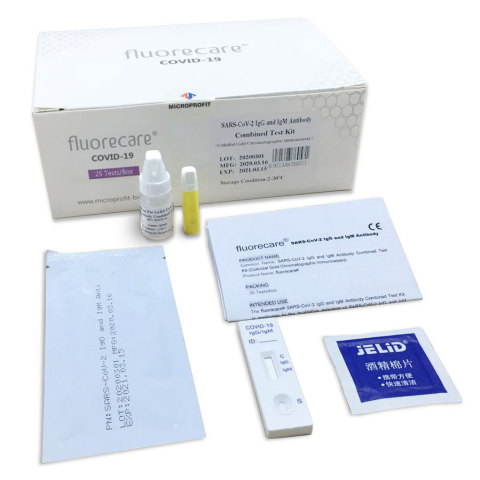
EMERYVILLE, Calif.--(BUSINESS WIRE)--NovaBay® Pharmaceuticals, Inc. (NYSE American: NBY) announces an agreement with Shenzhen Microprofit Biotech Co., Ltd. to become the exclusive U.S. distributor of a rapid, finger prick test to determine the presence of COVID-19 or a potential indication of antibody immunity to COVID-19. The fluorecare® SARS-CoV-2 IgG & IgM Antibody Combined (colloidal gold chromatographic immunoassay) Test Kit is a point-of-care test to be administered by healthcare professionals. The test uses a drop of blood for the detection of COVID-19 antibodies with results available in approximately 10 minutes.
The fluorecare test kit has been validated through widely used RT-PCR testing to detect immunoglobulin M (IgM), which is the first antibody produced in response to initial exposure to the COVID-19 antigen, and immunoglobulin G (IgG), which provides a potential indication of antibody-based immunity to COVID-19. The fluorecare test kit has been ISO 13485 and CE Mark certified.
“Public health experts and leaders across our country are citing a critical need for mass testing and tracing procedures for those who are infected or have been infected with COVID-19 before reopening the nation’s economy,” said Justin Hall, NovaBay CEO. “Nasopharyngeal (back of the nose and throat) swabs for molecular detection are expensive and require laboratory testing that can lead to delays in obtaining results. Through a simple finger prick, IgG/IgM testing could provide for cost-effective detection of COVID-19 antibodies with results available in minutes as an important step in tracking the infection.
“We are delighted once again to work with our global health supplier network to secure a product that can help our communities during the COVID-19 pandemic and, subject to FDA clearance, we plan to offer the fluorecare test kit at very competitive pricing,” he added.
NovaBay will submit the fluorecare test kit to the U.S. Food and Drug Administration (FDA) under Emergency Authorization Use (EAU), which will be effective until the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostic tests for detection and/or diagnosis of COVID-19 is terminated. The Company will also submit the fluorecare test kit for permanent FDA 510(k) clearance so that the test kit can continue to be used once the state of emergency has been declared over by the Federal Government. Because these test kits are one of the first few test kits of its kind to be reviewed by the FDA, NovaBay cannot assure a timeline for FDA review and/or clearance for commercial marketing of the fluorecare test kit in the U.S. under EAU or 510(k), or if clearance will be granted at all.
About NovaBay Pharmaceuticals, Inc.: Going Beyond Antibiotics®
NovaBay Pharmaceuticals, Inc. is a biopharmaceutical company focusing on commercializing and developing its non-antibiotic anti-infective products to address the unmet therapeutic needs of the global, topical anti-infective market with its two distinct product categories: the NEUTROX® family of products and the AGANOCIDE® compounds. The Neutrox family of products includes AVENOVA® for the eye care market, CELLERX® for the aesthetic dermatology market, and NEUTROPHASE® for wound care market.
Forward-Looking Statements
This release contains forward-looking statements that are based upon management’s current expectations, assumptions, estimates, projections and beliefs. These statements include, but are not limited to, statements regarding our ability to obtain both short and long term FDA approval for the test kits and the impact the test kits may have on our future financial results. These statements involve known and unknown risks, uncertainties and other factors that may cause actual results or achievements to be materially different and adverse from those expressed in or implied by the forward-looking statements. Factors that might cause or contribute to such differences include, but are not limited to, risks and uncertainties relating to consumer acceptance of our new branding, and any potential damage to our established goodwill in the marketplace. Other risks relating to NovaBay’s business, including risks that could cause results to differ materially from those projected in the forward-looking statements in this press release, are detailed in NovaBay’s latest Form 10-Q/K filings with the Securities and Exchange Commission, especially under the heading “Risk Factors.” The forward-looking statements in this release speak only as of this date, and NovaBay disclaims any intent or obligation to revise or update publicly any forward-looking statement except as required by law.
Socialize and Stay informed on NovaBay’s Progress
Like us on Facebook
Follow us on Twitter
Connect with NovaBay on LinkedIn
Visit NovaBay’s Website
Contacts
NovaBay Contact
Justin Hall
CEO and General Counsel
510-899-8800
jhall@novabay.com
Investor Contact
LHA Investor Relations
Jody Cain
310-691-7100
jcain@lhai.com