Imricor Announces First Cases Successfully Performed at Heart Center Dresden
Imricor Announces First Cases Successfully Performed at Heart Center Dresden
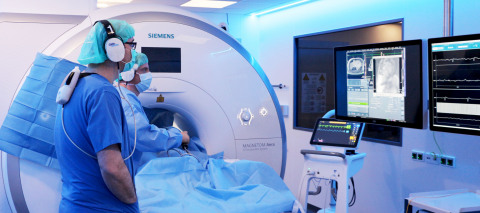
MINNEAPOLIS & DRESDEN, Germany--(BUSINESS WIRE)--Imricor Medical Systems, Inc. (Company or Imricor) (ASX:IMR) is pleased to announce the first procedures using the Company’s products following CE mark of the Vision-MR Ablation Catheter have been performed at the Heart Center Dresden.
Three procedures were successfully performed over two days by Dr. Christopher Piorkowski and Dr. Thomas Gaspar (https://www.herzzentrum-dresden.com/rhythmologie/unser-team/). Additional physicians at the Heart Center Dresden will also begin performing procedures immediately.
These procedures mark the first iCMR ablations anywhere in the world to ever be performed outside of a clinical trial. They were performed in an iCMR lab fitted with a Siemens 1.5T MAGNETOM Aera MR scanner.
“It’s another tremendous milestone for Imricor,” said Steve Wedan, Imricor’s Chair and CEO. “It marks the beginning of routine clinical use and the coming of age for this exciting new field of iCMR.”
Active Catheter Imaging
Procedures were performed, using native MR imaging on the Siemens scanner, which the Company calls Active Catheter Imaging. With this imaging technique, one can easily identify the Vision-MR Ablation Catheter without the use of active tracking or a mapping system.
Active Catheter Imaging proved to be highly effective in the procedures undertaken at Heart Center Dresden. Dr. Christopher Piorkowski commented during a procedure, “This is beautiful. It is better than fluoroscopy. In fluoroscopy you can only imagine the anatomy. Here you see it.”
Nick Twohy, Director of Marketing stated, “To complete our first cases is very exciting. We were ready to begin immediately after receiving approval. In addition to being commercially available, the Active Catheter Imaging technique enables healthcare professionals to provide care to patients today while at the same time preparing for advancements in 3D mapping technology on the horizon.”
About Imricor
Please visit http://imricor.com/investors/about-imricor/ for more information about Imricor, Foreign Ownership Restrictions, and Forward-Looking Statements.
Contacts
Investors:
Steve Wedan
Executive Chair, President and CEO
Email: steve.wedan@imricor.com
Carrie Barrack
Senior Advisor, Cato & Clegg
Email: carrie@catoandclegg.com
Phone: +61 422 464 028
Rest of World Media:
Nick Twohy
Director of Marketing, Imricor
Email: nick.twohy@imricor.com
Phone: +1 952 818 8407
Australia Media:
Carrie Barrack
Senior Advisor, Cato & Clive
Email: carrie@catoandclive.com
Mobile: +61 422 464 028