DENVER--(BUSINESS WIRE)--TriSalusTM Life Sciences (“TriSalus”), a company committed to transforming outcomes for patients with solid tumors, today announced the launch of its TriNav™ Infusion System (“TriNav”). Powered by its proprietary Pressure-Enabled Drug DeliveryTM (PEDD™) approach with SmartValveTM technology, TriNav is designed to help overcome the infusion barriers that limit therapeutic uptake in solid tumors, including hepatocellular carcinoma (HCC) and liver metastases.2,3
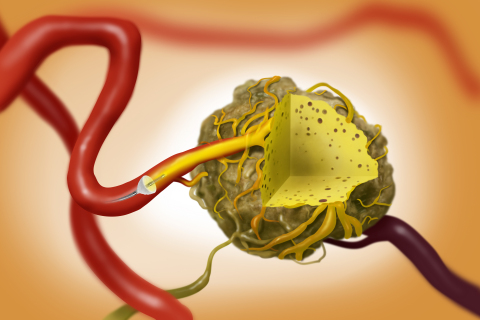
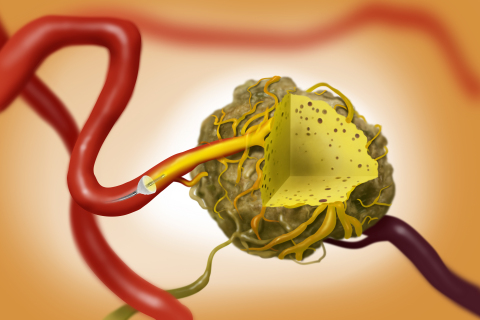
The TriNav System can provide interventional radiologists intravascular tumor access with improved trackability, compatibility with standard angiographic catheters and workflow comparable to standard interventions.4
The tumor microenvironment creates pressure barriers that limit flow into solid tumors.5,6 PEDD with SmartValve creates a high-pressure gradient that improves delivery and penetration of therapy into tumors.7,8 The porous expandable SmartValve is designed to allow antegrade flow and leverages blood flow to carry the dose deep into the solid tumor.1,7 SmartValve enables therapy delivery to the intended target while minimizing non-target delivery that has been shown to damage healthy tissue.2,9
In a clinical study, PEDD with SmartValve demonstrated the ability to overcome tumor infusion barriers and significantly improve response rates in HCC.2 Outcomes from a retrospective, single-center study of patients with solitary HCC tumors who underwent treatment utilizing either PEDD or standard end-hole microcatheters, showed 100% Objective Response (OR) with PEDD versus 76.5% with standard end-hole microcatheters (p=0.019).2* Additionally, after first treatment, Pathological Response (PR) as shown by tumor necrosis percentage was significantly greater with PEDD (88.8%) vs. standard end-hole microcatheters (33.8%), (p=0.026).2* Improving response rates could potentially help more patients meet transplant criteria, lead to successful downstaging, bridging, and post-transplant survival.10
“Tumor-directed delivery of therapeutics is an exciting opportunity to help improve outcomes across a wider range of procedures by overcoming intratumoral pressure that can prevent drugs from adequately penetrating the tumor,” said Mary Szela, President and CEO of TriSalus. “The new TriNav Infusion System utilizes SmartValve™, a first-in-kind, proprietary technology that has been shown to modulate pressure and flow with the goal for improved therapeutic delivery and deeper penetration into the tumor while helping to protect healthy tissue.”1-3,7,8
Acknowledging its unique technology for therapeutic delivery, the Centers for Medicare and Medicaid Services (CMS) granted TriNav transitional pass-through payment as part of the 2020 Medicare Hospital Outpatient Prospective Payment System and Ambulatory Surgical Center Payment System (CMS-1717-FC), effective January 1, 2020.12
This supplemental payment is intended to “facilitate access for [Medicare] beneficiaries to the advantages of new and innovative devices”13. The TriNav Infusion System met the required criteria to receive transitional pass-through status, including the demonstration of substantial clinical improvement, with the CMS saying it believes there is no existing pass-through payment category for this device because its SmartValve technology offers a unique mechanism for therapy delivery to selected sites in the peripheral vascular system, including solid tumors in the liver.12
*Study design: A retrospective, single-center study included 88 treatment-naive patients with solitary HCC tumors <6.5 cm who underwent treatment utilizing either PEDD™ (n = 18) or standard end-hole (EH) microcatheters (n = 70). Explant liver assessment of pathological response after first treatment: PEDD n=4; EH n=12.
About the TriNav™ Infusion System
The TriNav Infusion System is a 0.021-inch lumen microcatheter with SmartValve™ self-expanding tip. SmartValve supports pressure generation, which enables delivery of therapeutic agents to select sites in the peripheral vascular system, including solid tumors in the liver.2,3,8,14
The TriNav SmartValve has demonstrated the potential to overcome intratumoral pressure in solid tumors to improve distribution and penetration of therapy during arterial embolization procedures per clinical studies performed to date. 2,3,14
Intended Use: The TriNav Infusion System is intended for use in angiographic procedures. It delivers radiopaque media and therapeutic agents to selected sites in the peripheral vascular system.15
Contraindications: TriNav is not intended for use in the vasculature of the central nervous system (including the neurovasculature) or central circulatory system (including the coronary vasculature).15
For more information, please visit www.trinavinfusion.com.
About TriSalusTM Life Sciences
TriSalusTM Life Sciences is dedicated to improving patient outcomes in pancreatic and other highly intractable solid tumors. TriSalus Infusion Systems have the potential to deliver diagnostic, therapeutic agents, and immuno-stimulants directly into tumor vasculature powered by its proprietary Pressure-Enabled Drug DeliveryTM (PEDD™) approach with SmartValve™ technology to improve the distribution and penetration of therapy in solid tumors. This innovative approach has the potential to allow for the administration of multiple mechanisms that can work together to overcome inherent immune suppression within the solid tumor microenvironment.
For more information, please visit www.trisaluslifesci.com.
References
- Data on file (510K). TriSalusTM Life Sciences, 2019.
- Titano JJ, et al. Cardiovasc Intervent Radiol. 2019;42:560-568.
- Pasciak AS, et al. J Vasc Interv Radiol. 2015;26:660-669
- TriSalusTM TriNavTM Infusion System, Instructions for Use.
- Jain RK. Sci Am.2014;310:46-53.
- Sheth RA, et al. J Vasc Interv Radiol.2013;24:1201-1207.
- O’Hara R. Cardiovasc Intervent Radiol.2018 Mar; 41(Suppl 2):17-49. FOI: 10.1007/s00270-017-1852-5.
- Durham ED, et al. J Vasc Interv Radiol. 2015;26:e54(Poster 18).
- van den Hoven AF, et al. Cardiovasc Intervent Radiol. 2014;37:523-528.
- DiNorcia J, et al. Ann Surg. 2019. DOI: 10.1097/SLA.0000000000002381.
- Kim BK, et al. J of Heptol. 2015;62(6);1304-1310. DOI: https://doi.org/10.1016/j.jhep.2015.01.022.
- CY 2020 Medicare Hospital Outpatient Prospective Payment System and Ambulatory Surgical Center Payment System Final Rule.
- CMS. “Process and Information Required to Apply for Additional Device Categories for Transitional Pass-Through Payment Status Under the Hospital Outpatient Prospective Payment System.” March 2016. https://www.cms.gov/Medicare/Medicare-Fee-for-Service Payment/HospitalOutpatientPPS/Downloads/catapp.pdf
- Kim AY, et al. PloS One. 2017;12(9):e0183861. DOI: 10.1371/journal.pone.0183861
- TriSalus™ TriNav™Infusion System, Instructions for Use