GMA with MOVIVA®: setting new standards in endoscopic bariatric treatment
GMA with MOVIVA®: setting new standards in endoscopic bariatric treatment
The innovative approach works from the inside of the stomach to help reduce hunger and to support weight loss
TUEBINGEN, Germany--(BUSINESS WIRE)--Just a few months after the world’s first Gastric Mucosal Ablation (GMA) case using MOVIVA® at the Policlinico Universitario Agostino Gemelli in Rome, clinical experience is rapidly expanding across Europe. With around 80 procedures performed within a short timeframe in eight countries, MOVIVA® is helping to establish a new era of endoscopic bariatric treatment – offering a less invasive option as obesity rates continue to rise worldwide.
A promising alternative for bariatric patients: GMA with MOVIVA®
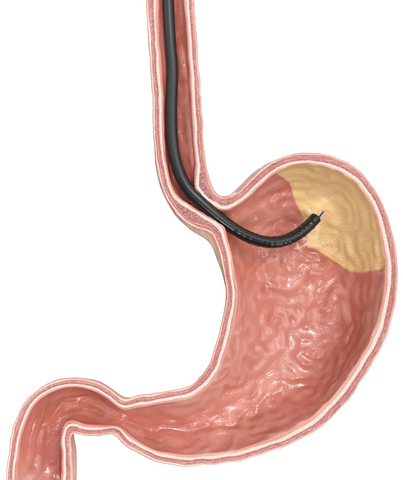
GMA is an incisionless, minimally invasive endoscopic procedure. It targets the gastric fundus, the main site of ghrelin production – the so-called hunger hormone. By reducing ghrelin levels, patients experience less hunger. At the same time, the ablated tissue shrinks the fundus, which reduces stomach capacity. Together, these effects can support controlled weight reduction.
MOVIVA® is a dedicated hybrid probe developed specifically for GMA. It combines high-pressure, needle-free injection to create a protective submucosal fluid cushion with superficial thermal ablation via argon plasma coagulation (APC). MOVIVA®’s dedicated moveAPD mode ensures broad and consistent argon plasma coagulation. Another strength of MOVIVA® lies in its enhanced injection capabilities, particularly in tangential positions, which are often challenging in endoscopic settings.
According to Prof. Ivo Boskoski, who led the first case in Rome: “Minimally invasive endoscopic techniques that target appetite regulation through precise ablation of the gastric fundus offer patients an effective pathway to weight loss without the risks associated with surgery. MOVIVA®’s innovative injection and ablation capabilities simplify the GMA procedure and could set a new standard in endoscopic bariatric care."
GMA can be performed as a stand-alone treatment or in combination with Endoscopic Sleeve Gastroplasty (ESG). In combination with ESG, the approach achieves weight loss outcomes comparable to vertical sleeve gastrectomy, while being less invasive.
“MOVIVA® sets new standards by addressing ghrelin, the hunger hormone, and has been shown to effectively manage hunger and cravings, supporting weight loss in people living with obesity. We are really looking forward to the commercial launch of MOVIVA® in 2026,” says Marcus Felstead, Chief Commercial Officer at Erbe Elektromedizin.
MOVIVA® is currently available in CE and CE-related markets.
Register for the free webinar "Advancing bariatric endoscopy"
Join Prof. Ivo Boskoski, MD, Christopher McGowan, MD and Abdullah AlMousa, MD on 11 December for a live online session on the latest developments in bariatric endoscopy and GMA with MOVIVA®: Webinar Registration - Zoom [https://erbe-med.zoom.us/webinar/register/WN_10nhAgt8TlOR4eu5qf51ZQ#/registration].
About Erbe Elektromedizin
Erbe Elektromedizin develops, manufactures, and distributes surgical instruments and devices worldwide, while providing services for professional use of these products in a diverse range of medical disciplines. Surgeons, OR teams and patients around the world rely on Erbe medical technology. The surgical instruments and devices find use in almost all specialist areas. They are based on electrosurgery combined with other Erbe technologies. Hybrid solutions enable us to provide new, innovative applications in medicine.
Fields of activity
- Imaging
- Endoscopy
- Electrosurgery
- Plasma surgery
- Thermofusion
- Hydrosurgery
- Cryo technology
An international network
- 20 international sales and service units
- 5 production sites
- Erbe is active in 110 international markets.
The Erbe workforce
- 2,100 employees worldwide
- Some 1,100 of them in Germany
Contacts
Erbe Elektromedizin GmbH
Waldhoernlestrasse 17
72072 Tuebingen
erbegroup.com
info@erbegroup.com
Editorial contact
Lisa Viergutz
Phone +49 7071 755-2731
lisa.viergutz@erbegroup.com