Worldwide First: Biedermann Motech Further Expands its MOSS!MODULARITY Platform and Announces Introduction of Multiple New Products
Worldwide First: Biedermann Motech Further Expands its MOSS!MODULARITY Platform and Announces Introduction of Multiple New Products
SCHWENNINGEN, Germany--(BUSINESS WIRE)--Biedermann Motech, the prominent innovator in next generation spinal and extremity implant systems and procedural solutions, today announced the market introduction of several additions to its MOSS!MODULARITY™ platform.
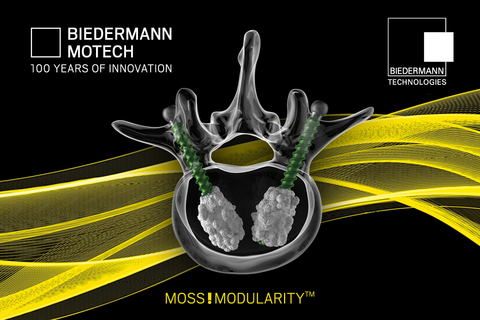
MOSS!MODULARITY encompasses a broad range of products and technologies for spinal surgery based around the concept of providing an advanced modular workflow for posterior stabilization and deformity correction. The modular workflow allows surgeons to place the headless bone screw shafts as a first step, without the tulip heads attached, and offers several advantages over working with traditional pre-assembled pedicle screws, e.g.:
- Improved visualisation and access to the surgical working space;
- Precise decompression, decortication and osteotomies without any interference with polyaxial tulip heads;
- Increased surgical versatility, as surgeons can choose between different types of modular screw shafts and various tulip heads to be added to the screw shafts in-situ as and when required.
Breaking new ground, Biedermann Motech will now introduce the MOSS!MODULARITY Injection Screw to selective markets outside the U.S. Fenestrated injection pedicle screws for spinal surgery, which Biedermann Motech has been pioneering since 2003, enable the injection of bone cement through the screw to enhance fixation in compromised bone1. The new injection screw workflow allows surgeons to insert the headless screw shaft directly into the bone, using the injection adaptor as the screw inserter, and then seamlessly inject bone cement before attaching the modular tulip heads to the screw shaft. The MOSS!MODULARITY Injection Screw workflow features proprietary technology licensed from intellectual property powerhouse and recognized innovation leader Biedermann Technologies GmbH & Co. KG and will be the first of its kind worldwide.
Simultaneously, Biedermann Motech will launch the next generation of its DELTA XS ® Retractor System for advanced Transforaminal Lumbar Interbody Fusion (“TLIF”) surgery. The DELTA XS 2.0 Retractor System has been specifically developed to combine the advantages of a screw shank based retractor with the benefits of the MOSS!MODULARITY workflow and can be used in a variety of surgical techniques and approaches, from open to less invasive, microsurgical TLIF surgery.
The MOSS!MODULARITY Injection Screw and the DELTA XS 2.0 Retractor System are fully compatible with the MOSS 100 Modular Pedicle Screw System, the MOSS VRS System and the MOSS VRS MIS System for advanced posterior fixation and correction and will further strengthen these platforms for specialized spinal surgery.
Biedermann Motech will showcase the worldwide first MOSS!MODULARITY Injection Screw and the DELTA XS 2.0 Retractor System at the upcoming Eurospine Meeting in Copenhagen, Denmark.
ABOUT BIEDERMANN MOTECH & BIEDERMANN TECHNOLOGIES
Biedermann is a mid-sized, family-owned group of companies, whose roots go back to 1916. Biedermann researches, develops, manufactures, and distributes high-end implant systems in collaboration with healthcare professionals, technology partners, scientific institutions, and specialized companies with the goal of achieving improved clinical outcomes. It is also owner and guardian of a substantial patent and technology portfolio for specialized orthopedic markets. For the last 30 years, Biedermann has successfully licensed numerous patents and related technologies to several key players in the orthopedic and neurosurgical field and established multiple new golden standards for spinal technologies.
For more information, please visit:
www.biedermann.com and www.biedermann-technologies.com
1 J Orthop Traumatol. 2011 Nov 8;12(4):193–199; Int J Spine Surg. 2021 Feb 26;15(1):113–118.