Kardium Announces First Commercial Procedures with the Globe® Pulsed Field System, the World’s Most Advanced Solution for Atrial Fibrillation
Kardium Announces First Commercial Procedures with the Globe® Pulsed Field System, the World’s Most Advanced Solution for Atrial Fibrillation
This milestone marks the commercial launch of the company’s next-generation system
VANCOUVER, British Columbia--(BUSINESS WIRE)--Kardium, a private medical device company that’s advancing the way the world treats atrial fibrillation (AF), today announced the successful completion of the first commercial procedures using its integrated mapping and ablation system, the Globe Pulsed Field System, following recent approval from the U.S. Food and Drug Administration (FDA).
The inaugural U.S. procedures were performed by Dr. Devi Nair, Chief of Cardiac Electrophysiology and EP Research at St. Bernards Medical Center in Jonesboro, Arkansas. “We are very excited to offer our patients access to this groundbreaking new technology,” said Dr. Nair. “The Globe Pulsed Field System gives me greater confidence that each lesion is delivered safely and effectively, to support better outcomes for my patients while also simplifying the procedure.”
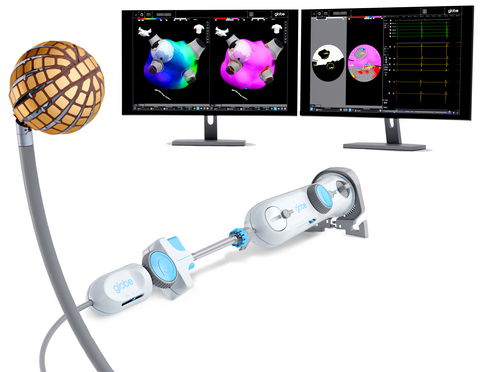
Combining high-density mapping and ablation into a single catheter enables the Globe System to deliver precise, targeted ablation using thermal contact assessment and tailored electrode selection. These capabilities allow for single-shot pulmonary vein isolation, and other customizable lesion sets, helping physicians as they strive for better outcomes for patients with atrial fibrillation.
“These first commercial procedures with the Globe System signal the beginning of a new era in AF treatment,” said Kevin Chaplin, CEO of Kardium. “We are proud to partner with leading physicians like Dr. Nair to bring the Globe Pulsed Field System into clinical practice allowing us to deliver a more personalized treatment for atrial fibrillation to improve long-term outcomes for patients.”
Data from the pivotal PULSAR study of the Globe Pulsed Field System, presented at the 2025 Heart Rhythm Society annual meeting, showed 78% freedom from atrial arrhythmia at one year in patients with paroxysmal AF patients, no device-related primary safety events and 95% lesion durability.
Following these successful first commercial procedures, Kardium will continue to expand commercial availability of the Globe Pulsed Field System across the United States, in collaboration with leading electrophysiology centers.
About Kardium
We are a rapidly growing, privately held medical solutions company with a mission to deliver the best treatment for atrial fibrillation through the Globe Pulsed Field System.
Contacts
T: +1.604.248.8891
E: Info@kardium.com