Global Cell and Gene Therapy Trials: Driving Innovation in Oncology and Beyond
Global Cell and Gene Therapy Trials: Driving Innovation in Oncology and Beyond
SAN FRANCISCO--(BUSINESS WIRE)--Novotech, the global full-service clinical Contract Research Organization (CRO), has published its latest whitepaper, offering in-depth analysis and emerging trends and advancements within the rapidly evolving field of cell and gene therapies. The report provides critical insights into the growth of clinical trials, innovations and strategic opportunities, shaping the future of this field.
Key Highlights from the Report:
-
Global Clinical Trial Expansion:
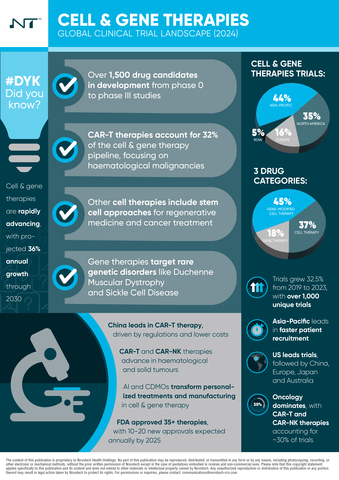
- Over 1,500 drug candidates in development across phases 0 to III.
- CAR-T therapies account for 32% of the pipeline, primarily targeting hematological cancers.
- The global trial landscape experienced a 32.5% growth from 2019 to 2023, with Asia-Pacific leading in trial activity (44% of all trials).
-
Oncology Focus:
- CAR-T and CAR-NK therapies represent ~30% of all cell and gene trials, with a strong focus on solid and hematological malignancies.
- Significant attention on gastrointestinal tract and lung cancers, as well as hematological cancers like diffuse large B-cell lymphoma (DLBCL).
-
Geographic Leadership:
- Asia-Pacific: Strong growth driven by China, which leads with 56% of the region's cell and gene trials.
- North America: The U.S. dominates with 85% of the trials.
- Europe: The UK, Spain, and Germany are key players.
-
Technological Advancements:
- AI and Contract Development and Manufacturing Organizations (CDMOs) are transforming the cell and gene therapy landscape, improving manufacturing efficiency and trial scalability.
-
Patient Recruitment Trends:
- Faster recruitment in Asia-Pacific (16.07 months) compared to the U.S. (21.15 months), with the region showing higher enrollment efficiency.
-
Investment Growth:
- A significant rise in venture capital for cell and gene therapies peaked in 2021, with sustained funding for start-ups and growth-stage companies.
Novotech’s whitepaper highlights the significant potential of cell and gene therapies, particularly in oncology, and outlines opportunities for increased collaboration in global clinical trials.
Download the full report to explore the complete clinical landscape and discover how Novotech can accelerate your research efforts.
About Novotech Novotech-CRO.com
Founded in 1997, Novotech is a global full-service clinical Contract Research Organization (CRO) focused on partnering with biotech companies to accelerate the development of advanced and novel therapeutics at every phase.
Recognized for its industry-leading contributions, Novotech has received numerous prestigious awards, including the Frost &Sullivan 2024 Global Biotech CRO of the year award, Clinical Trials Arena Excellence Awards 2024, 2024 Employer of Choice, 2024 Great Place to Work in the US, 2024 Brandon Hall Gold Award, CRO Leadership Award 2023, the Asia Pacific Cell & Gene Therapy Clinical Trials Excellence 2023, the Asia-Pacific Contract Research Organization Company of the Year Award since 2006.
The Company offers a comprehensive suite of services including laboratories, Phase I facilities, drug development consulting, regulatory expertise, and has experience with over 5,000 clinical projects, including Phase I to Phase IV clinical trials and bioequivalence studies. With a presence in 34 office locations and a dedicated team of 3,000+ professionals worldwide, Novotech is a trusted end-to-end strategic partner of choice.
For more information or to speak to an expert team member visit www.Novotech-CRO.com
Contacts
Media Contact
Toyna Chin
mediacontact@novotech-cro.com
USA: +1 415 364 8135