MMI Completes First Symani® Clinical Cases in the U.S. Following FDA Commercial Authorization
MMI Completes First Symani® Clinical Cases in the U.S. Following FDA Commercial Authorization
First in-human cases in the U.S. mark the start of a new era in soft tissue open surgery
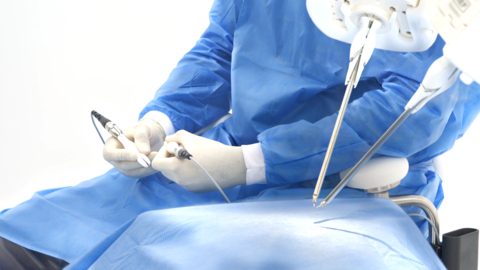
JACKSONVILLE, Fla.--(BUSINESS WIRE)--MMI (Medical Microinstruments, Inc.), a robotics company dedicated to increasing treatment options and improving clinical outcomes for patients with complex conditions, today announced the completion of its first clinical cases in the United States using the Symani® Surgical System. Both robotic-assisted microsurgical procedures were performed at Penn Medicine, Penn Presbyterian Medical Center, as a collaborative effort between the Department of Orthopaedic Surgery and the Department of Surgery’s, Division of Plastic Surgery.
The historic first U.S. procedures were reconstructive extremity microsurgeries. In one case, the team performed a “free bone transfer” procedure on a patient who suffered a traumatic injury; the procedure involved transferring a segment of bone and skin from their leg to a damaged bone in their forearm. The surgical team revascularized the bone segment using the microsurgical robot to reconnect the tiny blood vessels and facilitate successful transfer. The second case involved a patient at risk for a leg amputation due to an infected knee prosthesis with soft tissue deficiency. The team repaired the severe knee wound with muscle and skin from the patient’s back and robotically reconnected the blood vessels to promote revascularization.
“The first U.S. cases are a paramount milestone in the global expansion of the Symani Surgical System, and we’re honored to have been able to work with Penn Medicine’s premiere orthopedic and plastic surgery departments to achieve it,” said Mark Toland, CEO at MMI. “Today marks the beginning of a new era in surgical innovation, as patients across the country with conditions that require complex microsurgical techniques, such as extremity reconstruction, autologous breast reconstruction post cancer resection and lymphedema repair, will now have expanded access to treatment options.”
The U.S. Food and Drug Administration (FDA) recently granted De Novo Classification to the Symani Surgical System, making it the only commercially available platform in the U.S. for reconstructive microsurgery. It uniquely addresses the scale and complexities of microsurgery and supermicrosurgery such as the anastomosis and suturing of small anatomical structures – like blood and lymphatic vessels – during open surgical procedures. By allowing surgeons to replicate the natural movements of the human hand at the micro scale, it can expand treatment options for patients in need of advanced surgical techniques. It is designed to help restore quality of life for more patients, accelerate the number of surgeons able to push the boundaries of complex procedures for delicate anatomy, and enable hospitals to expand their open surgical programs.
To learn more about MMI and the Symani Surgical System, visit MMI’s website here: https://mmimicro.com.
About MMI
MMI (Medical Microinstruments, Inc.) is on a mission to advance robotic technology that pushes the limits of soft tissue open surgery and opens new opportunities for surgeons to restore quality of life for more patients with complex conditions. The company was founded in 2015 near Pisa, Italy, and its proprietary Symani® Surgical System combines the world’s smallest wristed microinstruments with tremor-reducing and motion-scaling technologies to address significant unmet patient needs across the globe. This first-of-its-kind surgical robotic platform for open, soft tissue micro-level surgery can help address microvascular repair and lymphatic repair. In Europe, it also addresses peripheral nerve repair. The Symani System is authorized for use in the U.S. by the FDA and is a CE Marked medical device in Europe. MMI is backed by global investors including Fidelity Management & Research Company, Andera Partners, BioStar, Deerfield Management, Fountain Healthcare Partners, Panakès Partners, RA Capital, Sambatech, and Wellington Partners.
Contacts
Media Contact:
Dan Ventresca
Matter Health for MMI
MMI@matternow.com
Investor Relations Contact:
Lisa Croke
Lisa.Croke@mmimicro.com