Able Medical Announces 510(k) Clearance of New RIB System Indicated for Thoracic Repair
Able Medical Announces 510(k) Clearance of New RIB System Indicated for Thoracic Repair
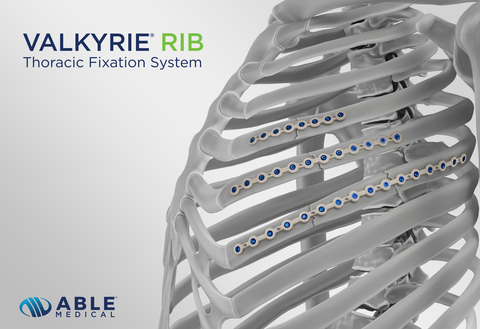
The single-use, PEEK-based RIB System utilizes the platform technology of Able’s Valkyrie® Thoracic Fixation System, further strengthening Able Medical’s portfolio.
MARQUETTE, Mich.--(BUSINESS WIRE)--Able Medical Devices has received U.S. FDA 510(k) clearance for its Valkyrie RIB System further enhancing its cardiothoracic and trauma portfolio. The novel single-use, PEEK device is an extension of Able’s Valkyrie Thoracic Fixation System and includes indications for the stabilization and fixation of fractures in the chest wall, reconstructive surgical procedures, trauma, and planned osteotomies.
“The Valkyrie RIB Thoracic Fixation System creates an adaptive fit to the bone, promoting closer contact with the underlying rib without the need for plate bending tools,” said Wade DePas, Senior Director of RAQA and R&D. “Supported by material science, this novel system is rooted in clinical history and prioritizes adaptability over the conventional norms of rigidity.”
Focusing on material science, the PEEK-based Valkyrie RIB System combines durable fixation with flexibility to help minimize patient stress shielding and conforms to meet various patient anatomies. The double-lead screws insert into the bone faster, increase bone purchase, and the “zero-chance” cross threading design reduces concern of screw back out.
“Ribs can elastically bend more than any other skeletal structure in the body, so offering a material that mimics native bone reduces the risk of stress shielding and offers several additional advantages over traditional metal plates,” said Peter J. Didyk, VP and Managing Director of Cardiothoracic and an inventor of the Valkyrie system. “Its minimal instrumentation focuses on speed, efficiency, and simplicity in the O.R. All of this, makes the Valkyrie RIB system a highly desirable product to the market.”
The Valkyrie RIB System is an exclusive member of the Valkyrie Thoracic Fixation family. The Valkyrie system is indicated for use in the stabilization and fixation of fractures in the chest wall including sternal reconstructive surgical procedures, trauma, or planned osteotomies. The system is intended for use in patients with normal and/or poor bone quality.
About Able Medical
Able Medical Devices is a subsidiary of J.M. Longyear and a leader in cardiothoracic products and contract manufacturing. Able Medical specializes in developing full surgical systems as well as custom instrumentation for the cardiothoracic market. The company’s extensive expertise includes working with surgeons for the cardiothoracic market as well as working with OEMs in spine and trauma. The Valkyrie® Thoracic Fixation System is a trademark of Able Medical.
Contacts
Jeffrey Gilhool, Senior Director of Marketing
(906) 373-9539
jeffreyg@abledev.us