CurvaFix to Host Industry Session at Orthopaedic Trauma Association Annual Meeting; Technology Featured in Poster Presentation
CurvaFix to Host Industry Session at Orthopaedic Trauma Association Annual Meeting; Technology Featured in Poster Presentation
Session to highlight how surgeons are using CurvaFix IM Implant for complex pelvic morphology, periprosthetic fracture, and Fragility Fractures of the Pelvis
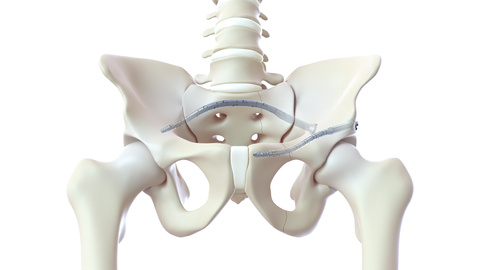
CurvaFix is hosting an Industry Session at the 39th annual meeting of the Orthopaedic Trauma Association (OTA), Oct. 18-21 in Seattle, highlighting how surgeons are using the CurvaFix Implant for complex pelvic morphology, periprosthetic fracture, and fragility fractures of the pelvis. (Graphic: Business Wire)
BELLEVUE, Wash.--(BUSINESS WIRE)--CurvaFix, Inc., a developer of medical devices that delivers stable fixation for curved anatomy and poor bone, has announced that it will host an Industry Session at the 39th annual meeting of the Orthopaedic Trauma Association (OTA), Oct. 18-21 in Seattle. Participants will learn how surgeons successfully utilize the CurvaFix® IM Implant, the only intramedullary implant that follows and fills the natural curvature of the pelvis, to treat patients with challenging pelvic fractures.
“Numerous case studies have shown that the CurvaFix Implant is able to overcome the limitations of existing implants used for pelvic fracture fixation in both high-impact trauma and fragility fracture patients.”
Share
CurvaFix also announced that a poster by Amir Matityahu, M.D., and additional authors outlining the results from a multicenter study evaluating the CurvaFix Implant will be presented at the annual meeting. The research, performed at seven centers in the U.S. and Canada, found that the CurvaFix Implant was safe and provided successful fixation of pelvic and acetabular fractures. The company will also showcase the CurvaFix procedure in booth #522 and provide updates on recent cases.
The Industry Session will occur on Thursday, Oct. 19, 7:00 am – 7:50 am (PT) in room 3B at the Seattle Convention Center, Arch Bldg. The educational session will include compelling case reviews, an overview of the CurvaFix technology, and panel discussions featuring Brett Crist, M.D., University of Missouri, Samir Mehta, M.D., Matthew Gardner, M.D., Springfield Clinic, John Michael Yingling, D.O., OU Health, Oklahoma, Prof. Dr. Pol M. Rommens, retired, University Medical Center of the Johannes Gutenberg-University of Mainz, Germany, and Langdon A. Hartsock, M.D., Medical University of South Carolina.
“With the aging population, osteoporosis is on the rise, making it imperative that surgeons have access to advanced technology that enables them to repair pelvic fractures that may cause significant pain and significantly limit patient mobilization,” said Dr. Crist. “The truly novel technology is minimally invasive, features outstanding intro-operative maneuverability, and has the potential for significant pain relief and faster mobility.”
Industry Session participants will hear how surgeons are utilizing the CurvaFix IM Implant to treat complicated pelvic morphology, periprosthetic fractures, and Fragility Fractures of the Pelvis (FFP). This event will include an update in early usage data, recent case presentations, and an evidence update on FFP.
“The CurvaFix Implant allows me to select the optimal surgical entry point and steer within the bone according to the patient’s anatomy,” said Dr. Mehta. “The result is durable, curved fixation—all in about 20 minutes. I see this as an optimal alternative solution for certain pelvic and acetabular fractures, especially with complex or atypical anatomy.”
“We’re thrilled to be hosting an Industry Session and presenting a poster at the OTA Annual Meeting, as we showcase the CurvaFix Implant, the most sophisticated technology to date for pelvic fracture repair,” said Mark Foster, chief executive officer of CurvaFix. “Numerous case studies have shown that the CurvaFix Implant is able to overcome the limitations of existing implants used for pelvic fracture fixation in both high-impact trauma and fragility fracture patients. We are focused on Restoring Mobility and Renewing Lives.”
About CurvaFix, Inc.
CurvaFix, Inc. is a privately held medical device company headquartered in Bellevue, Wash. CurvaFix is developing implantable products to improve fracture repair in curved bones. The company is focusing on Fragility Fractures of the Pelvis and high-impact pelvic fractures. The CurvaFix IM Implant has received 510(k) clearance from the U.S. Food & Drug Administration (FDA).
Contacts
Amy Cook
Acook@curvafix.com