Cell Reports Medicine Publication Demonstrates How Gelesis’ Oral Therapeutic Hydrogel Promotes Weight Loss and Metabolic Health by Targeting the Gut-Liver Axis in Pre-Clinical Studies
Cell Reports Medicine Publication Demonstrates How Gelesis’ Oral Therapeutic Hydrogel Promotes Weight Loss and Metabolic Health by Targeting the Gut-Liver Axis in Pre-Clinical Studies
Notably, hydrogel induced endogenous GLP1, reduced insulin resistance and improved insulin sensitivity, and prevented the progression of NAFLD
The proposed underling mechanism of action is regulation of gut barrier function and gut microbiota, to counter effects of high fat and high carbohydrate diets
A new paper published today suggests that weight loss resulting from Gelesis’ superabsorbent hydrogel treatment is not only a result of its space-occupying properties; the mechanical composition and structure of the material may have notable benefits for gut and metabolic health. (Graphic: Business Wire)
BOSTON--(BUSINESS WIRE)--A new paper published today in Cell Reports Medicine suggests that weight loss resulting from Gelesis’ superabsorbent hydrogel treatment is not only a result of its space-occupying properties; the mechanical composition and structure of the material may have notable benefits for gut and metabolic health.
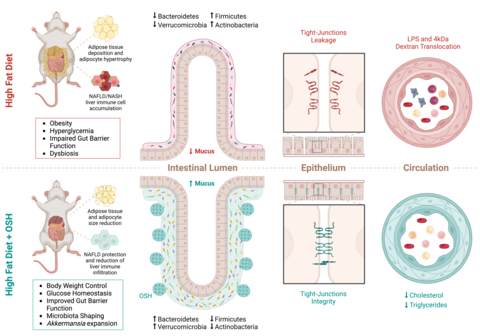
The continued rise in obesity and type 2 diabetes has led to the increasing prevalence of fatty liver disease worldwide and highlights the urgent need for therapies that can address the problem. Diets rich in fat, sugars, and ultra-processed foods are a contributor to rising obesity rates. These high fat/high carb diets have profound negative impacts on the host gut barrier function and microbial structure. This in turn has a detrimental effect and can lead to increased gut permeability and higher instances of metabolic disorders like obesity, diabetes, and fatty liver.
The reported studies examined both the efficacy and mechanism of action of Gelesis’ oral superabsorbent hydrogels (OSH) in preclinical models of diet-induced obesity, metabolic syndrome, and NASH. The Gelesis OSH consistently resulted in weight loss, improved insulin sensitivity, and prevented the progression of NAFLD.
Notably, the hydrogel induced endogenous GLP-1 and rapid, unique, and consistent changes in the gut microbiota, specifically fostering the growth of Akkermansia muciniphila, which is thought to be a beneficial microbe for metabolic disorders. Through comparison with supplement fiber (inulin, psyllium and hydrogels with different mechanical properties) it demonstrated that the physical structure of the hydrogel had the unique capability to boost Akkermansia growth. The study also demonstrated that the beneficial metabolic effects were independent of the weight loss effect.
“It was exciting to see how specific structural and physical properties are important for achieving the therapeutic effect on the gut microbiota, and how hydrogel-dependent preservation of the gut barrier properties is improving insulin sensitivity, promoting liver health and inducing weight loss,” said Maria Rescigno, PhD, professor at Humanitas University, group leader at Humanitas Research Hospital in Milan and senior author of the paper. “Our work shows the potential of oral superabsorbent hydrogels to be an effective and non-invasive therapeutic tool in the long-term treatment of obesity and metabolic disorders.”
Read the full paper in Cell Reports Medicine here.
About Gelesis
Gelesis Holdings Inc. (“Gelesis”) is a consumer-centered biotherapeutics company and the maker of Plenity®, which is inspired by nature and FDA cleared to aid in weight management. Our first-of-their-kind non-systemic superabsorbent hydrogels are made entirely from naturally derived building blocks. They are inspired by the composition and mechanical properties of raw vegetables, taken by capsule, and act locally in the digestive system, so people feel satisfied with smaller portions. Our portfolio includes Plenity® and potential therapies in development for patients with Type 2 Diabetes, Non-alcoholic Fatty Liver Disease (NAFLD)/Non-alcoholic Steatohepatitis (NASH), and Functional Constipation. For more information, visit gelesis.com, or connect with us on X, formerly known as Twitter: @GelesisInc.
Plenity® is indicated to aid weight management in adults with excess weight or obesity, a Body Mass Index (BMI) of 25–40 kg/m², when used in conjunction with diet and exercise.
Important Safety Information about Plenity
- Patients who are pregnant or are allergic to cellulose, citric acid, sodium stearyl fumarate, gelatin, or titanium dioxide should not take Plenity.
-
To avoid impact on the absorption of medications:
- For all medications that should be taken with food, take them after starting a meal.
- For all medications that should be taken without food (on an empty stomach), continue taking on an empty stomach or as recommended by your physician.
- The overall incidence of side effects with Plenity was no different than placebo. The most common side effects were diarrhea, distended abdomen, infrequent bowel movements, and flatulence.
- Contact a doctor right away if problems occur. If you have a severe allergic reaction, severe stomach pain, or severe diarrhea, stop using Plenity until you can speak to your doctor.
Rx Only. For the safe and proper use of Plenity or more information, talk to a healthcare professional, read the Patient Instructions for Use, or call 1-844-PLENITY.
Forward-Looking Statements
Certain statements, estimates, targets and projections in this press release may constitute “forward-looking statements” within the meaning of the federal securities laws. The words “anticipate,” “believe,” continue,” “could,” “estimate,” “expect,” “intend,” “may,” “might,” “plan,” “possible,” “potential,” “predict,” “project,” “should,” “strive,” “would” and similar expressions may identify forward-looking statements, but the absence of these words does not mean that statement is not forward looking. Forward-looking statements are predictions, projections and other statements about future events that are based on current expectations and assumptions and, as a result, are subject to risks and uncertainties. Forward-looking statements include, but are not limited to, statements regarding Gelesis’ or its management team’s expectations, hopes, beliefs, intentions or strategies regarding the future, including those relating to Gelesis’ expected operating and financial performance and market opportunities. In addition, any statements that refer to guidance, projections, forecasts, or other characterizations of future events or circumstances, including any underlying assumptions, are forward-looking statements. Forward-looking statements speak only as of the date they are made. Readers are cautioned not to put undue reliance on forward-looking statements, and Gelesis assumes no obligation and does not intend to update or revise these forward-looking statements, whether as a result of new information, future events, or otherwise. Gelesis gives no assurance that any expectations set forth in this press release will be achieved. Various risks and uncertainties (some of which are beyond Gelesis’ control) or other factors could cause actual future results, performance or events to differ materially from those described herein. For a description of such factors, please see the section entitled "Risk Factors" in Gelesis’ most recent Annual Report on Form 10-K and in other filings that Gelesis makes with the Securities and Exchange Commission. These filings address important risks and uncertainties that could cause actual results and events to differ materially from those contained in the forward-looking statements.