NEW YORK--(BUSINESS WIRE)--Paige, the global leader in AI-based diagnostic software in pathology, today launched its latest product, Paige Breast Lymph Node, an AI medical device software that helps pathologists detect if breast cancer has metastasized to lymph nodes, concurrent with pathologists’ own interpretive review*. The product was unveiled at the United States and Canadian Academy of Pathology (USCAP) Annual Meeting taking place March 19-24, 2022.
Determining whether cancer in the breast has spread to the lymphatic system is critical to a patient’s diagnosis and planned treatment. Due to the size of the tissue area and size of the small micro-metastases, a pathologist’s accurate assessment of a lymph node slide can be tedious and time-consuming.
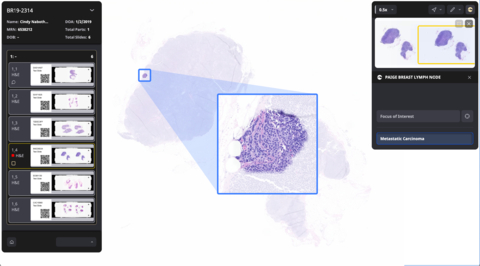
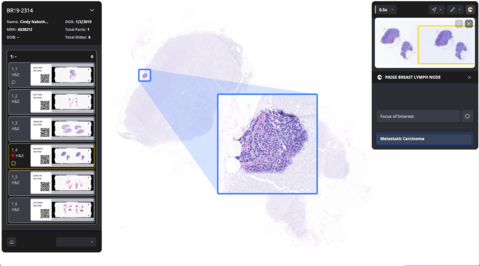
Designed to increase the accuracy and efficiency in the detection of breast cancer metastases that are most at-risk for being missed, Paige Breast Lymph Node leverages AI to empower pathologists to identify tumor metastases of any size, including small micrometastases, more efficiently and reliably. It is designed to enhance diagnostic accuracy for the most subtle metastatic foci and has over 98% slide level sensitivity to detect metastases of any size. A positive indication of metastatic breast cancer will be displayed at the individual lymph node level with Paige’s proprietary TissueMap, which highlights all regions on a slide that are suspicious for cancer. In addition, both slides and cases with suspected positive lymph nodes are highlighted, facilitating their prioritization and efficient review by the pathologist.
“Accurate detection of breast cancer metastases is paramount for physicians and their patients, but it can be a laborious, manual task for pathologists,” said David Klimstra, M.D., Founder and Chief Medical Officer at Paige. “Paige Breast Lymph Node offers pathologists a quicker and more efficient way to analyze large quantities of lymph node tissue, as well as peace of mind for them and their patients.”
Paige Breast Lymph Node will be available immediately as part of the Paige Breast Suite. This software uses the same underlying AI technology as Paige Prostate, which can work with a broad range of data, staining techniques, and scanning artifacts, resulting in a generalizable AI that can be quickly deployed in a variety of laboratory and hospital settings.
“Paige Breast Lymph Node bolsters the value of the Paige Breast Suite to clinical pathologists,” said Andy Moye, Ph.D., Chief Executive Officer at Paige. “This product launch is an important step in our overall commercialization strategy as we bring the power of our AI platform to new disease areas. Alongside our FDA-approved Paige Prostate, the generalizability of the AI further validates the broader use of Paige’s software in assisting pathologist to diagnose cancer.”
Paige will host a webinar on March 30 to demonstrate how Paige Breast Lymph Node can be used in clinical practice. For more information about Paige Breast Lymph Node, visit http://info.paige.ai/breast or contact info@paige.ai.
*Paige Breast Lymph Node is available for research use only in United States and in other regions where research use is permitted. Research use only products should not be used in diagnostic procedures.
About Paige
Paige was founded in 2017 by Thomas Fuchs, Dr.Sc., David Klimstra, M.D., and colleagues from Memorial Sloan Kettering Cancer Center (MSK). The company builds computational pathology products designed so patients and their care teams can make effective, more informed treatment decisions. With this new class of AI-based technologies positioned to drive the future of diagnostics, Paige created a platform to deliver this novel technology to pathologists to transform their workflow and increase diagnostic confidence and productivity. Paige’s products deliver insights to pathologists and oncologists so they can arrive efficiently at more precise diagnoses for patients. Paige is the first company to receive FDA approval for an AI-based digital pathology product.
For additional information, please visit: https://www.paige.ai, Twitter and LinkedIn.