VUZE Medical Announces New Worldwide Patents
VUZE Medical Announces New Worldwide Patents
Real-time Image Processing Enables Software-Only Guidance and Verification in Minimally Invasive Spine Surgery
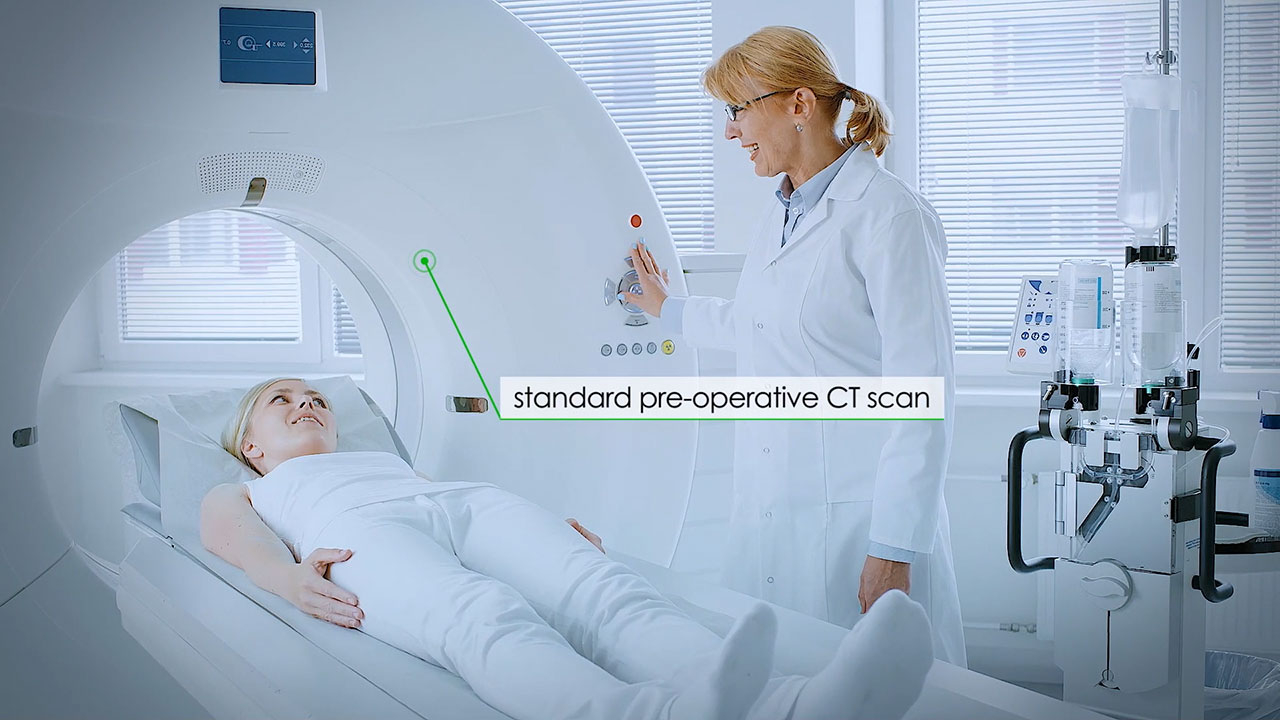
The VUZE System is a software-only solution that overlays a graphical representation of tools seen in intra-operative 2D images onto axial and sagittal views generated from the patient’s standard pre-operative 3D scan. The system is designed for use during common spinal surgeries that are performed in outpatient or ambulatory settings. Watch how it works in this video.
RA’ANANA, Israel--(BUSINESS WIRE)--VUZE Medical, a privately held medical technology company aimed at transforming image guidance and verification in minimally invasive spine surgeries, has received additional patents for its VUZE™ System in the U.S., Europe and China. Using proprietary image processing, the VUZE System is a software-only solution that overlays a graphical representation of tools seen in intra-operative 2D images onto axial and sagittal views generated from the patient’s standard pre-operative 3D scan. FDA 510(k) clearance was granted in January 2022.
Chinese Patent No. 109195527B and European Patent No. 3429475 follow previously issued U.S. Patent No. 10716631 in describing the core workflow of the VUZE System. Additionally, U.S. Patent No. 11224483 describes a unique form of 2D-3D image co-registration via machine learning in which the learning phase is performed solely upon the 3D scan of the same patient of whom 2D images are later acquired.
“These new patents further establish our intellectual property as one of the unique strengths of the VUZE solution,” said David Tolkowsky, VUZE Medical’s CEO. “We believe that our continued commitment to investing in IP will translate in the near future into further patents granted worldwide.”
Currently in clinical validation, the VUZE System is designed for use during common spinal stabilization surgeries that are performed in outpatient or ambulatory settings and are currently aided solely by X-ray. The system uses no sensors, cameras or reference arrays whatsoever, nor does it require any calibrations or lines of sight. It can be used with standard surgical tools and implants with no tool add-ons or modifications.
More than three million surgeries for correcting spinal instability and/or deformation, collectively known as spinal stabilizations, are performed annually worldwide, with a third of those in the U.S.1 These procedures include vertebral fixation with pedicle screws, vertebral fixation coupled with fusion, and vertebral augmentation with synthetic or biological cement. Approximately 80 percent of stabilizations treat short spinal segments 1. Short-segment surgeries are most often performed manually and are typically assisted only by standard 2D X-ray.
About VUZE Medical
VUZE Medical is a privately-held medical technology company that aims to provide highly accurate and cost-effective surgical guidance for common spinal interventions currently aided only by standard 2D X-ray alone. The company’s VUZE System is a unique software-only solution that instantly merges intra-operative X-ray with pre-operative CT, providing surgeons with the cross-sectional images they need most during surgery and currently lack. The system is designed for use during common spinal surgeries that are performed in outpatient or ambulatory settings. For more information, visit www.vuzemedical.com.
1. Orthopedic Network News: 2020 Spinal Surgery Update
Contacts
Joe Duraes
Pazanga Health Communications
917-687-6419
jduraes@pazangahealth.com
