SurGenTec Launches ION Screw, Smallest Posterior Spinal Fixation Implant, to Market After Receiving FDA Clearance
SurGenTec Launches ION Screw, Smallest Posterior Spinal Fixation Implant, to Market After Receiving FDA Clearance
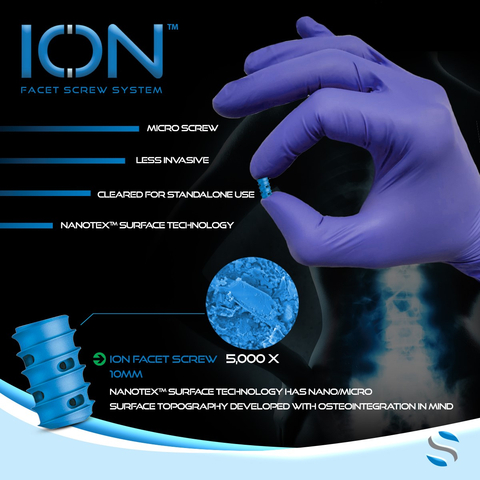
BOCA RATON, Fla.--(BUSINESS WIRE)--SurGenTec®, a privately held spine and orthopedic technology company based in Boca Raton, FL, announced that it has received clearance from the U.S. Food and Drug Administration (FDA) for ION™ Screw, its proprietary stand-alone spine fixation implant. This unique device can be used to treat a variety of pathologies throughout the spine from C2-S1. The patented ION implant is the smallest posterior fixation implant cleared for spine surgery – smaller than the size of a dime – and can be placed through a tiny incision with minimal tissue disruption. ION screws are enhanced with Nanotex™ surface technology designed with bony interdigitation in mind. ION provides patients with a treatment option between conservative care and major interbody fusion in the algorithm of care.
“The ION screw technology truly changes the game for spine surgery. Patients are looking for options outside traditional bulky metallic devices such as pedicle screws and interspinous plates. This minimally invasive option provides excellent fixation with zero profile. It’s a non-intimidating solution for patients that need to undergo a spine surgery procedure. We are thrilled to announce the addition of ION to our portfolio. Our team has put years into pre-clinical research and development to create this truly novel spinal fixation option,” said Travis Greenhalgh, Founder and Chief Executive Officer of SurGenTec. “Our team managed to create a ‘micro’ implant with a unique surface technology that provides fixation without the need for cumbersome implants,” added Greenhalgh.
ION can be used in a variety of open or true minimally invasive surgery (MIS) applications including facet fusions, adjunct to decompressive procedures, the treatment of adjacent level disease or to provide added stability with interbody fusions. The ION facet screw system instruments were designed to minimize tissue trauma and incision size while delivering the ION implants to the surgical location. "The ION system is a great fit for the ambulatory surgery center environment by providing a cost-effective solution for physicians looking to minimize trauma while still providing a high level of spinal care to patients. Patients are demanding access to a more efficient operating environment with less risk of illness and disease transmission," said Andrew Shoup, Chief Operating Officer of SurGenTec.
“ION represents a tremendous paradigm shift in the ability to treat my patients suffering from chronic degenerative lumbar conditions more definitively and less invasively,” said Dawood Sayed M.D., Division Chief and Professor of Anesthesiology and Pain Medicine at The University of Kansas Medical Center in reference to the recent FDA cleared ION facet screw system. “Traditionally, our patients with chronic back pain from lumbar degenerative disc disease, spondylosis, and spondylolisthesis are offered temporary relief with injections and ablations or invasive open surgical procedures. ION bridges an important gap for patients and physicians seeking less invasive options for these debilitating conditions. I am very honored to be amongst the first in the world to offer this remarkable treatment at our medical center,” continued Sayed.
Unlike other spinal fixation options, the ION has zero profile above the bone which may prevent soft tissue contact and the potential for irritation. The implants may be packed with bone graft prior to and after insertion to help facilitate fusion while providing an opportunity for bone to bridge over the implant and prevent implant migration.
“Using the ION system was intuitive and safe. It allowed me to provide a less invasive solution than I would have typically provided. The standard of care is changing, and ION offers a simple solution to advanced spinal conditions,” said Dr. Raphael Roybal M.D., orthopedic spine surgeon Coral Gables, FL.
SurGenTec is a privately owned medical device company based in Boca Raton, Florida that is focused on unique technologies for the orthopedic and spine fields. SurGenTec develops and manufactures innovative products with patient and surgeon safety in mind. SurGenTec looks to launch ION immediately, with plans in the future of obtaining CE mark to market overseas. The vast pipeline of unique implants, instruments, biologics and ancillary solutions will continue to develop through a high level of internal research and development. SurGenTec currently has multiple products lines to market which are sold within the US and internationally. In 2018 and 2020, SurGenTec received the Spine Technology Awards for their ALARA™ target needle system and 3D Graftrasp presented at NASS (North American Spine Society).
For more information on ION, visit www.SurGenTec.com or contact customerservice@surgentec.com.
Contacts
SurGenTec
Ricki Goldman, 561-990-7882