Pearl’s Second Opinion® Introduces Groundbreaking AI-Assisted Radiology to Canadian Dentistry
Pearl’s Second Opinion® Introduces Groundbreaking AI-Assisted Radiology to Canadian Dentistry
Canada has joined a growing list of countries where Pearl’s flagship dental AI solution has been licensed for distribution.
LOS ANGELES--(BUSINESS WIRE)--Pearl, the leader in AI solutions for efficiency, accuracy, and consistency in dental care, today announced that it has received a Medical Device Establishment License (MDEL) for its Second Opinion® AI radiology solution from Canada Health. Second Opinion previously received CE-marking in Europe and TGA/MEDSAFE clearance in Australia and New Zealand for aiding dentists in their detection of a broad range of dental conditions via x-rays in accordance with patient and user safety standards.
The latest approval in Canada further indicates the integral role AI can play within the dental industry. Pearl was founded to bring transparency and objectivity to dentistry by applying computer vision to deliver solutions that improve consistency in radiologic interpretation, among other crucial tasks. Second Opinion® offers dentists an assistive tool to use when interpreting x-rays, helping build confidence in treatment recommendations for dentists and patients alike.
"Patients in Canada now have the opportunity to experience the benefits of AI-assisted dentistry first hand,” said Ophir Tanz, CEO and founder of Pearl. "Quality dental treatment starts with quality radiology and Second Opinion® allows dentists to optimize their works as radiologists, enabling them to consistently deliver need-based care of the highest standard.”
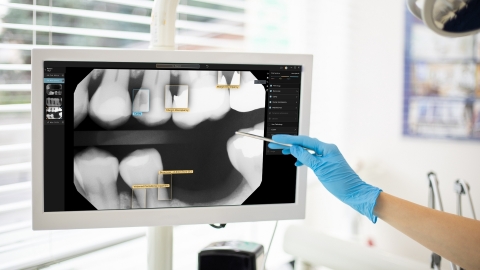
Second Opinion® applies computer vision technologies to detect a comprehensive array of pathologies, existing restorations and natural anatomy in dental radiographs. The system analyzes radiographs in real time, allowing dentists to display, review, add or reject the system’s detections while conducting exams in the operatory.
Dentists in Canada and other approved territories can begin using Pearl’s Second Opinion® today by visiting: www.hellopearl.com/products/second-opinion.
About Pearl
Pearl is shaping the future of dental care by delivering AI and computer vision solutions that advance efficiency, accuracy, transparency and patient care. Founded in 2019 by Ophir Tanz, Pearl is backed by Craft Ventures and other leading venture capital firms. For more information or to request a demonstration, please visit https://www.hellopearl.com.
Contacts
Alex Foley
Matter Health for Pearl
Pearl@matternow.com