During the Covid-19 Pandemic, San Francisco-Based CRO ClinPro Trials Works With Sponsors Throughout Their Drug Development Cycle From Concept to Commercialisation
During the Covid-19 Pandemic, San Francisco-Based CRO ClinPro Trials Works With Sponsors Throughout Their Drug Development Cycle From Concept to Commercialisation
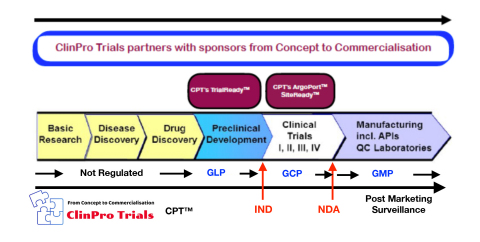
SAN FRANCISCO--(BUSINESS WIRE)--With the current Covid-19 pandemic crisis, the world is painfully aware of the criticality for life saving drugs to be developed and brought to market rapidly. For a drug to be brought to market, the sponsor company works with a Clinical Research Organisation (CRO) who manages the clinical trials as the drug goes through its various development Phases from the Concept to the Commercialisation Stage. A good CRO is invaluable to a sponsor company in its pursuit to bring a new drug to market, and a poor performing CRO can cause the regulatory approval for a new drug to be denied or delayed. ClinPro Trials (CPT) www.clinprotrials.com is a One Stop full service CRO that operates on five continents and works with sponsors to help them forge new paths to develop and commercialise their therapies. CPT offers Pre-Clinical and Clinical Trial Research expertise to sponsors throughout the drug development cycle from the drug’s Concept to Commercialisation. It is headquartered in San Francisco, where it works with sponsors across the globe.
“Through preparing and hosting Pre-Approval Inspections for sponsors for over a decade, we observed that the lacklustre service and products provided by most CROs during the conduct of clinical trials, resulted in sponsors being subjected to negative regulatory actions during the Pre-Approval Inspection process which resulted in either the new drug approval being delayed or denied. CPT is a game changer for the CRO industry and we stand behind our services and products,” said the CEO of ClinPro Trials, Monita Dukhia. “At CPT we do not regard sponsors as just another account; we are committed to building long lasting relationships with our clients who are on the cusp of delivering life changing therapies to patients. CPT has raised the bar in how PreClinical and Clinical CROs services are offered in the healthcare industry and sponsors are taking notice,” she continued.
CPT is transforming the manner in which CRO services are offered to sponsors in the following ways:
- CPT’s core competency is compliance and the services and products it delivers to sponsors will withstand the scrutiny of any Regulatory Inspections. Other CROs exit the clinical study once the Phase 3 trial is completed which leaves the sponsor to grapple with preparing and hosting the Pre-Approval Inspection; whereas CPT can continue to work with the sponsor to prepare them for, and host the Pre-Approval Inspection.
- By offering CPT TrialReady™ which delivers PreClinical expertise to empower sponsors to move rapidly from the drug Concept stage into the Clinic. This platform enables sponsors to progress laboratory research discoveries to clinical proof of concept effectively translating preclinical molecules to promising clinical therapies.
- By offering CPT SiteReady™; a turn key operation which manages all aspects of Clinical Site operations from Site Identification to Site Close out
- By offering CPT ArgoPort™ ; a Digital Patient Recruitment Platform which enables sponsors to meet patient enrolment targets
CPT has strong regional experience throughout Europe, Russia, Asia, Australia and the US. The CRO has strategically established eight branches globally to meet the needs of sponsors. Your therapy is of paramount importance to patients, so you need a high-performance CRO partner who will deliver what they promise and stand behind their services. CPT is the rational choice for the CRO who will work with you throughout your drug development cycle from Concept to Commercialisation.
Contacts
Monita Dukhia: mdukhia@clinprotrials.com
Phone: 415 350 9536