Medable Releases Global TeleVisit Capabilities to Enable Continued Clinical Trial Research in Light of Growing COVID-19 Concerns
Medable Releases Global TeleVisit Capabilities to Enable Continued Clinical Trial Research in Light of Growing COVID-19 Concerns
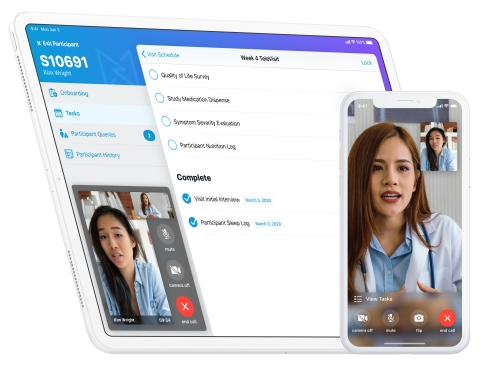
PALO ALTO, Calif.--(BUSINESS WIRE)--In response to the global COVID-19 pandemic, Medable has developed a new TeleVisit mobile application that can be used by patients worldwide to connect virtually with their clinical trial sites. The application makes it possible for clinical trial research to progress in an environment where many patients are being told to stay home and avoid social interaction. The application includes the following capabilities:
- Consents patients in their native language for the use of telemedicine;
- Provides real-time video capabilities on a single platform that is 21 CFR, Part 11 compliant;
- Allows patients to virtually connect with their site coordinators, investigators or other care professionals from anywhere in the world;
- Removes the burden and cost of travel for on-site clinical visits; and
- Increases patient safety in light of increasing COVID-19 infection rates.
The application is designed for rapid deployment in one week or less, and can be used immediately by existing clinical trial patients and sites.
“We need to reduce the risk for participants while also enabling research to move forward,” said Dr. Michelle Longmire, Co-Founder and CEO of Medable. “There are more than 50,000 active clinical trials underway. These studies are extremely important and many have been underway for years. We need to ensure participants can receive medical care and evaluation of endpoints during critical study time windows. TeleVisit and decentralized clinical trials enable research to continue while we combat the outbreak with containment measures. We have ensured that we can operationalize this infrastructure on a global scale to support research worldwide.”
Medable’s global TeleVisit application can be used for various decentralized trials and hybrid trials and works with patients’ existing mobile devices as well as tablets used by clinical trial sites. Alternatively, Medable can work with clinical trial teams to provision devices globally, including phones or tablets. Once users download the application, they can connect via one click to a live video stream to discuss personal health developments or concerns.
For more information on Medable’s Trial-Fit TeleVisit offering, contact COVID19@medable.com.
About Medable
Medable is on a mission to reduce clinical trial timelines by 50 percent. The company’s digital platform streamlines design, recruitment, retention and data quality for decentralized trials, replacing siloed systems with integrated digital tools, data and interfaces to accelerate trial execution. Medable connects patients, sites and clinical trial teams to improve patient access, experience, and outcomes. Medable is a privately held, venture-backed company headquartered in Palo Alto, California. For more information, visit www.medable.com and follow @Medableinc on Twitter.
Contacts
Lisa Barbadora, Big Valley Marketing for Medable, +1 (610) 420-3413, media@medable.com