Diality Announces First Patient Treated With Moda-flx Hemodialysis System
Diality Announces First Patient Treated With Moda-flx Hemodialysis System
Patient-inspired technology delivers lifesaving dialysis therapy
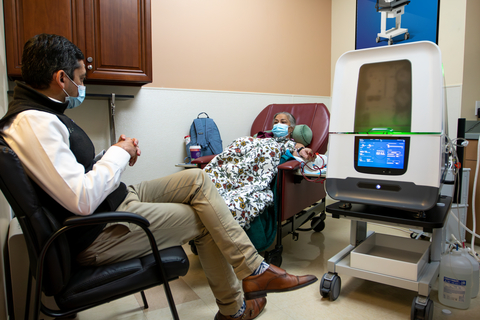
IRVINE, Calif.--(BUSINESS WIRE)--Diality, a medical device company developing a smart, flexible hemodialysis platform, today announced successful completion of the first-ever patient treatment with the Moda-flx Hemodialysis System™ in collaboration with North America Research Institute at Ontario-Holt Dialysis Center in Ontario, Calif.
“Our partnership with the North America Research Institute is an important step toward delivering on our promise to improve the lives of patients and families affected by kidney disease,” said Osman Khawar, M.D., CEO of Diality. “This is the first of many patients who will enjoy a better dialysis experience with the Moda-flx Hemodialysis System.”
The Moda-flx Hemodialysis System is designed to empower kidney care professionals with a wide range of variable flow rates, integrated reverse osmosis water filtration, and an intuitive, easy-to-use graphical user interface. The Moda-flx Hemodialysis System provides clinicians the flexibility to easily customize each hemodialysis experience according to patient needs in one integrated system. The platform’s compact footprint and mobility enable seamless integration and transportation within dialysis care settings.
“New kidney care technology, especially innovative technology such as the Moda-flx Hemodialysis System, which allows us to personalize dialysis prescriptions to the needs of our patients, is exactly what the industry needs,” said Dr. Aamir Jamal, CEO of North America Research Institute. “The clinical flexibility the Moda-flx Hemodialysis System offers dialysis providers is liberating. We can choose the right prescription for our patients and trust that the system will be able to deliver the therapy our patients need.”
The Moda-flx Hemodialysis System is indicated for use in patients with acute and/or chronic renal failure, with or without ultrafiltration, in an acute, post-acute, or chronic care facility. Treatments must be administered under a physician’s prescription, by a trained person who is considered competent in the use of the device. Treatment types available include: Intermittent Hemodialysis (IHD), Sustained Low Efficiency Dialysis (SLED/SLEDD), and Prolonged Intermittent Renal Replacement Therapy (PIRRT).
About Diality
Diality is a medical device company focused on developing solutions to improve lives impacted by kidney disease. The Moda-flx Hemodialysis System is a user-friendly, mobile, and connected hemodialysis platform designed to maximize clinical flexibility by combining prescription capabilities of in-center systems with the ease-of-use of next generation hemodialysis devices. Moda-flx Hemodialysis system obtained U.S. FDA clearance in August 2024. Please visit www.diality.com or find us on LinkedIn to learn more about Diality and The Moda-flx Hemodialysis System™.
About North America Research Institute
North America Research Institute provides innovative kidney care through clinical trials with its extensive network of office & dialysis locations throughout Southern California. North America Research Institute is dedicated to providing excellent and compassionate care to help their patients lead healthy lives. Please visit https://ctv.veeva.com/site/north-america-research-institute-nari-san-dimas-ca-dr-jamal to learn more about services and meet their team.
Contacts
MEDIA CONTACT:
Sam Choinski
Pazanga Health Communications
schoinski@pazangahealth.com
(860) 301-5058