Aerin Medical Announces Sustained Clinical and Quality-of-Life Improvements Two Years After Chronic Rhinitis Treatment with RhinAer®
Aerin Medical Announces Sustained Clinical and Quality-of-Life Improvements Two Years After Chronic Rhinitis Treatment with RhinAer®
The Largest Multi-Center Study of Radiofrequency PNN Ablation Confirms that Long-Term Outcomes are Reproduceable
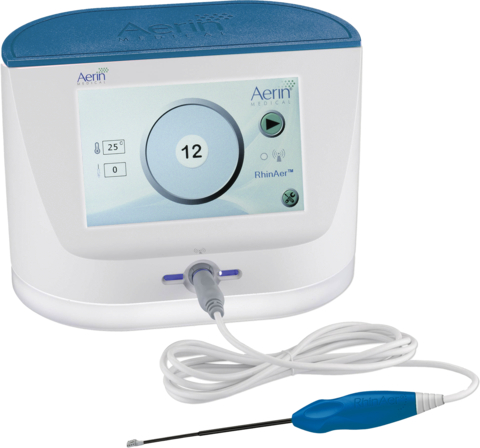
Two-year results of the RELIEF clinical trial published in The Laryngoscope confirm that a single procedure with RhinAer® resulted in sustained improvements in chronic rhinitis symptoms and quality of life, along with a substantial decrease in chronic rhinitis medication usage. RhinAer, a non-invasive technology developed by Aerin Medical Inc., uses temperature-controlled, radiofrequency energy to provide relief from chronic rhinitis. (Photo: Business Wire)
MOUNTAIN VIEW, Calif.--(BUSINESS WIRE)--Aerin Medical Inc., a company dedicated to providing Ear, Nose and Throat (ENT) physicians with non-invasive solutions for the treatment of chronic nasal conditions, today announced the publication of two-year positive results from the RELIEF clinical trial in The Laryngoscope. RELIEF demonstrated that a single procedure with RhinAer®, a temperature-controlled, radiofrequency technology, resulted in sustained improvements in chronic rhinitis symptoms, including post-nasal drip and chronic cough, and quality of life through two years along with a substantial decrease in chronic rhinitis medication usage.
“Publication of more peer-reviewed evidence reinforces Aerin Medical’s commitment to supporting the interests of otolaryngologists and their patients,” said Matt Brokaw, CEO of Aerin Medical.
Share
The prospective, single-arm RELIEF study, conducted across 16 centers in the United States and Germany, enrolled 129 patients seeking relief from chronic rhinitis symptoms. The targeted treatment area focused on the posterior nasal nerve (PNN) using RhinAer. Patients were asked to report post-nasal drip and chronic cough symptoms, quality of life evaluation and medication usage. Key findings of the study highlight the longevity of RhinAer’s treatment effect in all areas of measurement through two years, including:
- A significant improvement in reflective total nasal symptom score (rTNSS) sustained over two years.
- Significant improvement in chronic cough and post-nasal drip from baseline.
- A clinically relevant increase in quality of life for 77.4% of patients at the two-year mark as assessed by the mini rhinoconjunctivitis questionnaire.
These sustained improvements following treatment with RhinAer were also accompanied by a significant reduction in medication use. Of the 81 patients using chronic rhinitis medications at baseline, most either stopped or decreased use at the two-year mark. Furthermore, no device or procedure-related serious adverse events were reported throughout the two years.
“Medications are commonly prescribed for chronic rhinitis, but unfortunately often have limited effectiveness,” said Daniel D. Charous, M.D., Arizona Desert Ear, Nose & Throat Specialists and investigator for the RELIEF clinical study. “This study’s results reinforce existing evidence that a single procedure with RhinAer offers durable symptom relief and also reduces medication use for my patients.”
Rhinitis, or inflammation of the mucous membrane of the nose, can include symptoms such as runny nose, congestion, itching, sneezing, coughing, and post-nasal drip. Chronic rhinitis, when symptoms last more than four consecutive weeks, can be challenging to treat and may significantly lower a person’s quality of life. ENTs use RhinAer as a non-invasive alternative to surgical interventions to directly interrupt nerve signals and help reduce chronic rhinitis symptoms.
“Publication of more peer-reviewed evidence reinforces Aerin Medical’s commitment to supporting the interests of otolaryngologists and their patients,” said Matt Brokaw, CEO of Aerin Medical. “Chronic rhinitis is one of the most common conditions presenting to ENTs, and we’re grateful to physician investigators who have contributed to RhinAer’s body of evidence as the only radiofrequency device with established multi-year durability.”
Results of the RELIEF study add to Aerin Medical’s body of clinical evidence that supported a Category I Current Procedural Terminology (CPT®) code application established by the Centers for Medicare and Medicaid Services (CMS) that will be effective Jan. 1, 2024. The new code, which is for endoscopic destruction of the PNN using radiofrequency ablation, describes the procedure performed by ENT physicians when treating overactive nerves that drive chronic rhinitis symptoms with RhinAer.
About RhinAer
Using temperature-controlled, radiofrequency technology, RhinAer features a thin, wand-like stylus that is inserted via the nostril to deliver precise therapeutic benefits, while sparing surrounding tissues. RhinAer directly disrupts the posterior nasal nerve (PNN) that triggers excessive mucus production, treating an underlying cause of chronic rhinitis. RhinAer provides ENT physicians with a comprehensive solution for the treatment of chronic rhinitis, addressing sources of rhinorrhea (runny nose) and congestion. The procedure can be performed with a local anesthetic during an office visit, with no incisions, minimal to no downtime, and little discomfort. RhinAer initially received FDA 510(k) clearance in December 2019 and CE Mark in 2020. For more information, visit www.RhinAer.com.
About Aerin Medical
Aerin Medical is a privately held, venture-backed company, with U.S. offices in California and Texas. Aerin’s mission is to expand access to meaningful relief for millions of patients suffering from chronic ENT conditions. The company’s products, VivAer® for nasal airway obstruction and RhinAer® for chronic rhinitis, leverage Aerin’s proprietary temperature-controlled technology, which allows ENT physicians to reliably improve patients’ symptoms with unique technologies that are appealing alternatives to invasive surgery. More than 100,000 patients have been treated with Aerin Medical products to date. For more information, please visit www.aerinmedical.com and follow Aerin Medical on Facebook, X, Instagram, LinkedIn and YouTube.
Contacts
Laura Morgan
951-333-9110
laura@merrymancommunications.com