Rhinostics Selected to Exhibit RHINOstic® Automated Swab Platform at Vizient Innovative Technology Exchange
Rhinostics Selected to Exhibit RHINOstic® Automated Swab Platform at Vizient Innovative Technology Exchange
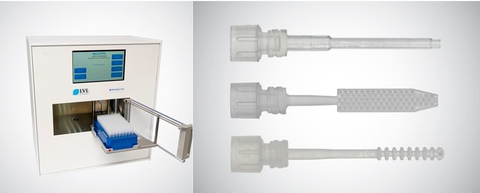
The RHINOstic® system enables the first handsfree swab diagnostic workflow, replacing manual procedures to bring swabs into assay workflows. The system integrates a stacked-ring polypropylene swab with an automated cap. After a patient sample is collected, the swab is placed into a collection tube for dry shipment, reducing sample loss during transport and accidental biohazard contact. When it reaches the lab, the RHINObot™ Decapper robotically removes the swab and links it to downstream liquid handlers and automated assay workflows. Rhinostics offers a growing family of automated sample collection devices including capillary blood and DNA collection, all targeted towards improving the patient collection experience and the laboratory med tech work environment while increasing efficiencies and turnaround times.
WALTHAM, Mass.--(BUSINESS WIRE)--Rhinostics has been selected to exhibit its RHINOstic® Automated Swab platform at the Vizient Innovative Technology Exchange. Vizient, Inc, the nation’s largest member-driven healthcare performance improvement company, will hold the Exchange on Oct. 3 in Grapevine, Texas.
The annual Innovative Technology Exchange offers selected suppliers the unique opportunity to demonstrate their product or service to supply chain and clinical leaders from Vizient’s member hospitals and subject matter experts who serve on their supply councils. Each product or service will showcase how it improves clinical outcomes, enhances safety or drives incremental improvements to health care delivery or business models.
The RHINOstic® system enables the first handsfree swab diagnostic workflow, replacing manual procedures to bring swabs into assay workflows. The system integrates a stacked-ring polypropylene swab with an automated tube cap to reduce human contact. After a patient sample is collected, the swab is placed into a collection tube for dry shipment, reducing sample loss during transport and accidental biohazard contact. When it reaches the lab, the RHINObot™ Decapper robotically removes the swab and links it to downstream liquid handlers and automated assay workflows. Globally, laboratories have used the swabs for COVID-19 testing, multi-analyte respiratory testing, vaginal and triple-site collection for sexually transmitted infections (STIs), wounds, and other swab sample types.
“We are honored to bring our technology platform to Vizient’s Innovative Technology Exchange. We believe our groundbreaking RHINOstic automated swab will positively impact their membership in many areas, including comfortable sample collection combined with the ability to streamline their laboratory workflows. Hospital systems continue to consolidate laboratories to serve an increasing number of locations. Each lab that adopts our technology quickly realizes the benefits of stream-lined workflows, more reliable and repeatable testing through robotics, reducing labor dependency and costs, and dropping their reagent usage to make a significant impact on their cost of goods. Increasingly, every laboratory is looking for ways to manage their laboratory staff turnover while managing costs more effectively,” said Cheri Walker, PhD, President and CEO of Rhinostics.
“Suppliers come to the Exchange hoping to be awarded an Innovative Technology contract, which signals health care providers of their product’s unique qualities,” said Kelly Flaharty, senior director of contract services, Vizient. “We are pleased to include this technology in the group selected to participate.”
The annual Innovative Technology Exchange is part of Vizient’s Innovative Technology Program that includes product review of supplier-submitted technologies by member-led councils and task forces. Since 2003, Vizient has reviewed over 1,600 product submissions as part of its Innovative Technology Program.
About Rhinostics
Rhinostics is at the forefront of revolutionizing sample collection. As a spin-out company from Harvard University and Wyss Institute, we are taking a radical new approach to sample collection device design and function. We recognize that many automated microplate-based workflows are encumbered by manual steps at the workflow’s beginning, and aim to develop and commercialize elegant, automated collection devices to break through these bottlenecks. Using our advanced collection device technologies such as the RHINOstic® and ELEstic™ Automated Swabs, VERIstic® blood collection devices and HIPPOstic™ DNA collection device, test kit manufacturers can empower comfortable yet high-end patient experiences with abundant and clean sample yields for tests from lateral flow to microfluidics; all while boosting profits and differentiating their brand. Test processing laboratories conducting sensitive assays from traditional PCR to LAMP or antigen testing and more gain increased sample throughput compared to manual workflows for cost-effective and robust assay performance even when working with difficult samples; with labor reductions even during times of surging demand. Rhinostics aids testing workflows in areas including respiratory disease, genomics, STDs, forensic and much more. Rhinostics continues to innovate, launching the three new collection devices and several robotic decapper systems with our partners to provide systems for our purpose-built sample collection devices for their application combined with automation enablement. Rhinostics products are registered as Class I exempt medical devices with the U.S. FDA and may be used for clinical collection upon CLIA validation. To learn more, visit https://www.rhinostics.com.
Contacts
Media and Investors:
Cheri Walker, Rhinostics, Inc.
(877) 746-6789
info@rhinostics.com