Dexcom G6 Real-Time CGM System Now Available to All Clients With Type 1 Diabetes Covered Under the Non-Insured Health Benefits Program
Dexcom G6 Real-Time CGM System Now Available to All Clients With Type 1 Diabetes Covered Under the Non-Insured Health Benefits Program
- Dexcom G6 was the first Continuous Glucose Monitoring (CGM) System to be officially listed by the NIHB Drug Benefit program.
BURNABY, British Columbia--(BUSINESS WIRE)--Dexcom, Inc. (NASDAQ: DXCM), the global leader in real-time continuous glucose monitoring (rtCGM), is pleased to announce that coverage for Dexcom G6 rtCGM System on the Non-Insured Health Benefits (NIHB) Program has been expanded to include all clients living with type 1 diabetes. This announcement expands upon coverage already in place for clients aged 2 to 19 on intensive insulin therapy, giving even more First Nations and Inuit people access to this standard of care, potentially helping them to have more control over a life-long chronic illness.
“Indigenous, First Nations and Inuit communities are disproportionately impacted by diabetes beyond childhood and adolescence, making this announcement a key step forward to ensuring better glucose control, improved health outcomes and reduced risk of developing diabetes-related complications,” said Dr. Jeff Winterstein, an Edmonton-based internal medicine specialist who works with many NIHB patients who live with diabetes. “Furthermore, CGM can help to bridge the distance between remote communities and care providers. The Dexcom CLARITY software allows me to remotely see a patient’s glucose data and trends over time so I can make the appropriate adjustments and treatment decisions.”
Dexcom rtCGM use is proven to improve glycemic control1,2 and can reduce the risk of costly long-term diabetes-related complications.3 As part of the NIHB coverage program, clients aged 2 to 19 on intensive insulin therapy AND clients of all ages with type 1 diabetes can now obtain their Dexcom G6 rtCGM supplies directly from their local pharmacy.
“CGM positively impacts the health1,2 of both pediatric and adult patients and expanded access is an important step forward in creating a more equitable healthcare system across the country,” says Laura Endres, Senior Vice President of Dexcom. “We applaud the NIHB for offering more people the chance to take control of their health and live better with diabetes.”
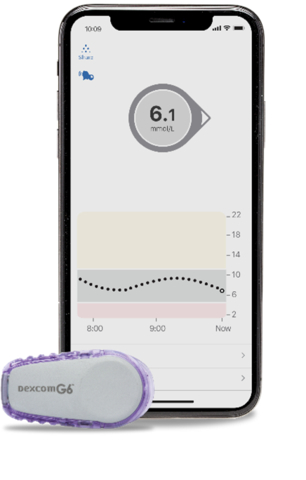
Dexcom G6 uses a small, wearable sensor and transmitter to continuously measure and send glucose levels wirelessly to a smart device* or receiver, giving patients real-time glucose data without the need to scan or prick their finger routinely.† The system has customizable and predictive alerts and alarms to help avoid potentially dangerous low and high blood sugar events and a function that allows patients to share their glucose data in real time with up to 10 followers.
Dexcom G6 also offers industry-leading connectivity through integrations with leading insulin delivery systems and digital health apps.
For more information on Dexcom G6, please visit https://www.dexcom.com/en-CA/en-ca-dexcom-g6-cgm-system
To search the online NIHB Drug Benefit List for Dexcom G6 coverage details, go to https://nihb-ssna.express-scripts.ca/en/0205140506092019/16/160407
About Dexcom, Inc.
Dexcom, Inc. empowers people to take control of diabetes through innovative continuous glucose monitoring (CGM) systems. Headquartered in San Diego, California in the United States, and with operations in Canada, Dexcom has emerged as a leader of diabetes care technology. By listening to the needs of users, caregivers, and providers, Dexcom simplifies and improves diabetes management around the world. For more information about Dexcom CGM, visit www.dexcom.com.
† If your glucose alerts and readings from the G6 do not match symptoms or expectations, use a blood glucose meter to make diabetes treatment decisions.
* For a list of compatible devices, please visit dexcom.com/compatibility
References
1 Beck RW, Riddlesworth T, Ruedy K, et al. Effect of continuous glucose monitoring on glycemic control in adults with type 1 diabetes using insulin injections: The DIAMOND randomized clinical trial. JAMA 2017;317(4):371-8.
2 Welsh JB, Gao P, Derdzinski M, et al. Accuracy, Utilization, and Effectiveness Comparisons of Different Continuous Glucose Monitoring Systems. Diabetes Technol Ther 2019;21(3):128-32.
3 Roze S, Isitt J, Smith-Palmer J, Lynch P. Evaluation of the Long-Term Cost-Effectiveness of the Dexcom G6 Continuous Glucose Monitor Versus Self-Monitoring of Blood Glucose in People with Type 1 Diabetes in Canada. Poster presentation presented at: 2020 Canadian Association for Population Therapeutics; October 27, 2020.
Contacts
Alena Atkinson, 705.206.9991
Veritas Communications
atkinson@veritasinc.com