Paragon 28 Launches TenoTac™ 2.0 Soft Tissue Stabilization System for Hammertoe and Soft Tissue Repair
Paragon 28 Launches TenoTac™ 2.0 Soft Tissue Stabilization System for Hammertoe and Soft Tissue Repair
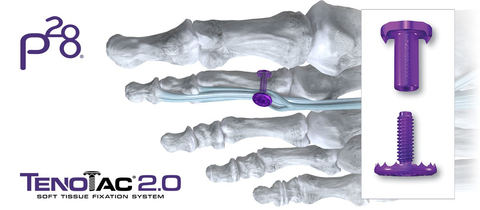
ENGLEWOOD, Colo.--(BUSINESS WIRE)--Paragon 28, Inc. (NYSE: FNA), a leading medical device company exclusively focused on the foot and ankle orthopedic market, today announced further expansion of its hammertoe and soft-tissue portfolio with the launch of its TenoTac™ 2.0 Soft Tissue Fixation System. The TenoTac™ 2.0 Soft Tissue Fixation System uses a titanium threaded implant and was designed to allow for greater capture of soft tissue, streamlined tensioning, and improved fit to the bony surface.
Paragon 28’s CEO, Albert DaCosta, commented, “With the launch of TenoTac™ 2.0, we further refined our initial offering to allow surgeons greater control and precision to address the unique needs of each patient. TenoTac™ 2.0 adds to our growing portfolio of soft tissue products and the Hammertoe segment, with opportunities for significant improvements in patient outcomes.”
The addition of the TenoTac™ 2.0 Soft Tissue Fixation System bolsters Paragon 28’s hammertoe product offering, which includes the Paratrooper™ Plantar Plate System, HammerTube™ Hammertoe System, Mini-Monster® Screw System, and JAWS Staple System. With this comprehensive portfolio, Paragon 28® provides its customers innovative forefoot solutions to address flexible and rigid contractures of the lesser toes.
About Paragon 28, Inc.
Based in Englewood, CO., Paragon 28, is a leading medical device company exclusively focused on the foot and ankle orthopedic market and is dedicated to improving patient lives. From the onset, Paragon 28® has provided innovative orthopedic solutions, procedural approaches and instrumentation that cover a wide range of foot and ankle ailments including fracture fixation, hallux valgus (bunions), hammertoe, ankle, progressive collapsing foot deformity (PCFD) or flatfoot, charcot foot and orthobiologics. The company designs products with both the patient and surgeon in mind, with the goal of improving outcomes, reducing ailment recurrence and complication rates, and making the procedures simpler, consistent, and reproducible.
Contacts
Investor Contact:
Gilmartin Group
Matt Bacso, CFA
matt.bacso@gilmartinir.com